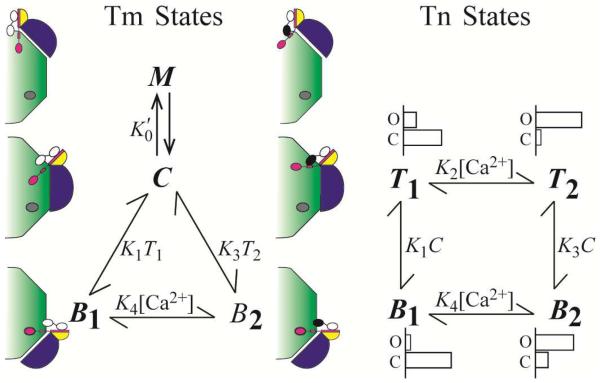
Figure 2. Model accounting for results of functional studies.
The TnIsp is modeled as binding either TnC or actin and TnC is modeled as being in either the closed or open conformation. The model consists of two subsystems that partially overlap. The states of Tm (blue) include C, M, and Bi (i = 1, 2), corresponding to interactions with actin (green) in positions central, myosin dependent, and blocking, respectively. Steady-state constant, , governs the ensemble dynamic interactions of myosin with Tm that sustain M. The states of Tn (TnT, yellow; TnI, magenta, TnC, black-white) include Ti and Bi, corresponding to energetically coupled and uncoupled in the Ca2+-free state (i = 1) and Ca2+-bound state (i = 2), respectively. Most probable occupancy of the N-terminal domain regulatory site of TnC is shown as Ca2+-bound (black) or Ca2+-free (white). Equilibrium constants govern spontaneous interactions that occur between Tn and actin (K1 and K3), and Ca2+ and Tn (K2 and K4); K1 and K3 also include the equilibrium that favors Tm in the C position. For the Tn states, representative distributions of closed and open conformations of TnC are diagramed to show the ratios 9/1, 1/3, 3/1, and 1/9 for B1, B2, T1, T2, respectively, which are intended for relative comparison only.