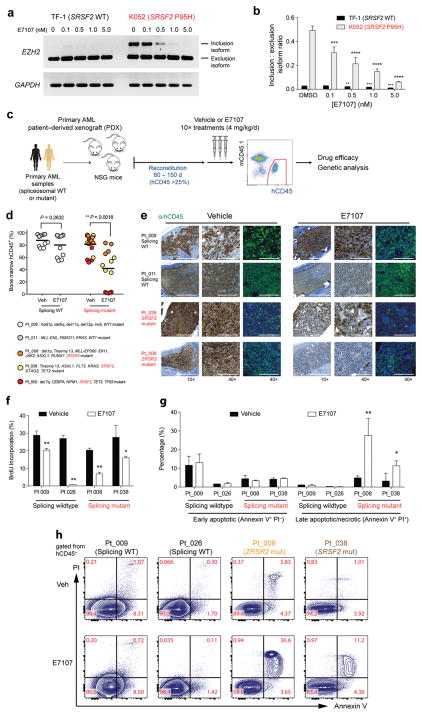
Figure 4. Preferential sensitivity of primary human leukemias to pharmacologic modulation of splicing in vivo with E7107.
(a) RT-PCR and (b) qRT-PCR analysis of the effect of E7107 exposure (6 hours) relative to DMSO treatment on expression of a cassette exon inclusion isoform (“poison” exon) of EZH2 in SRSF2 wildtype (TF-1) or mutant (K052) leukemia cell lines. (c) Schema of patient-derived xenograft (PDX) experiments using primary human acute myeloid leukemia (AML) samples wildtype or mutant for spliceosomal genes followed by engraftment into NOD-scid IL2Rnull (NSG) mice. (d) Percentage of BM human CD45 (hCD45) cells in NSG mice following 10 days of vehicle or E7107 (4 mg/kg/d) treatment based on spliceosome mutational status. Each point represents hCD45 values for one individual NSG mouse and each color represents PDX from a specific patient. Mutational data for each patient are listed below the graph. (e) Immunohistochemical and immunofluorescence analysis for hCD45 in BM sections of recipient mice as shown in (d) based on spliceosome mutational status. (f) Percentage of BM hCD45+ cells in S-phase based on in vivo BrdU-incorporation following 5 days of E7107 (4 mg/kg/d) or vehicle treatment. (g) Bar plots showing percentage of hCD45+ cells that are Annexin V+/PI−or Annexin V+/PI+. (h) Representative FACS plots of Annexin V/propidium iodide (PI) staining of hCD45 cells following 5 days of E1707 (4 mg/kg/d) or vehicle treatment in vivo. Error bars represent mean ± SD. *P < 0.05; **P < 0.01; ***P < 0.001; **** P <0.0001.