Figure 1.
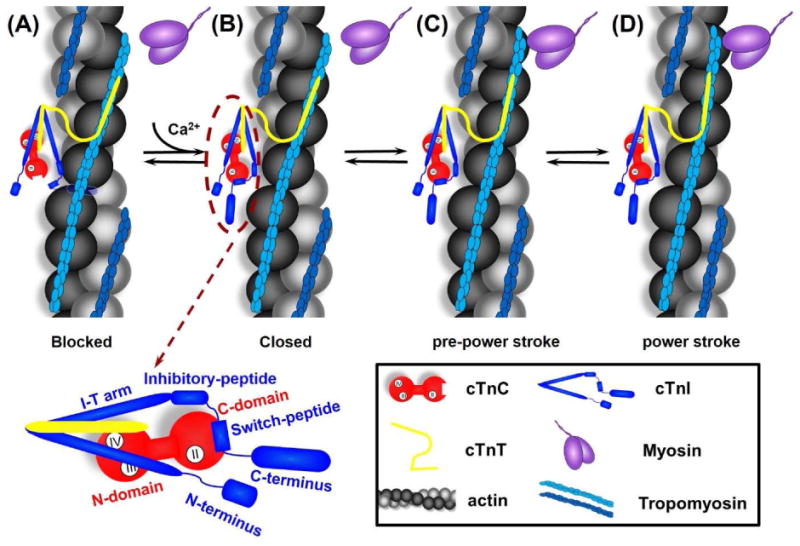
Cartoon model of the signaling pathway for thin filament activation. From left to right are: (A) In diastole (in the absence of Ca2+), cTnC exists in its “closed” conformation, and the inhibitory-peptide of cTnI binds actin tightly, inhibiting actin-myosin interaction. (B) In systole, Ca2+ binding to site II of cTnC induces an “open” conformation and increases interaction between NcTnC and cTnI switch-peptide, which pulls the adjacent inhibitory-peptide of cTnI from binding with actin. (C) Consequently, this leads to increased Tm mobility, exposes the myosin binding site on actin and myosin weakly interaction with actin (pre-power stroke), and (D) This weakly interaction further pushes Tm, and myosin strongly binds with actin, allowing the formation of cross-bridges and thus force generation (power stroke). The left-bottom is the close-up of cTn complex with the labeling of specific region of cTnC and cTnI.
