Figure 3.
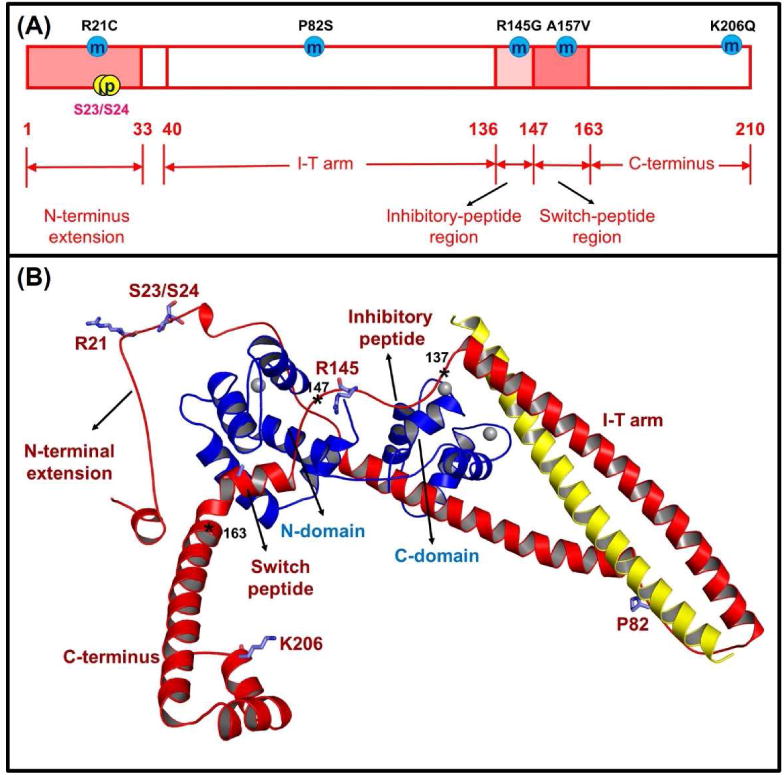
Human cTnI (A) sequence and (B) ternary structure with the some HCM-related mutation sites located at different regions of cTnI (R21C, P82S, R145G, A157V and K206Q) and PKA phosphorylation sites (S23/S24). In the ternary structure, the cTnC (1-161) is shown in blue, the cTnI (1-210) is in red, and the cTnT (236-285) is in yellow. The asterisks indicate three key positions in human cTnI, for which residues 137-146 is the inhibitory-peptide region of cTnI, residues 147-163 is the switch-peptide region of cTnI, and residues 164-210 belong to the C-terminus of cTnI.
