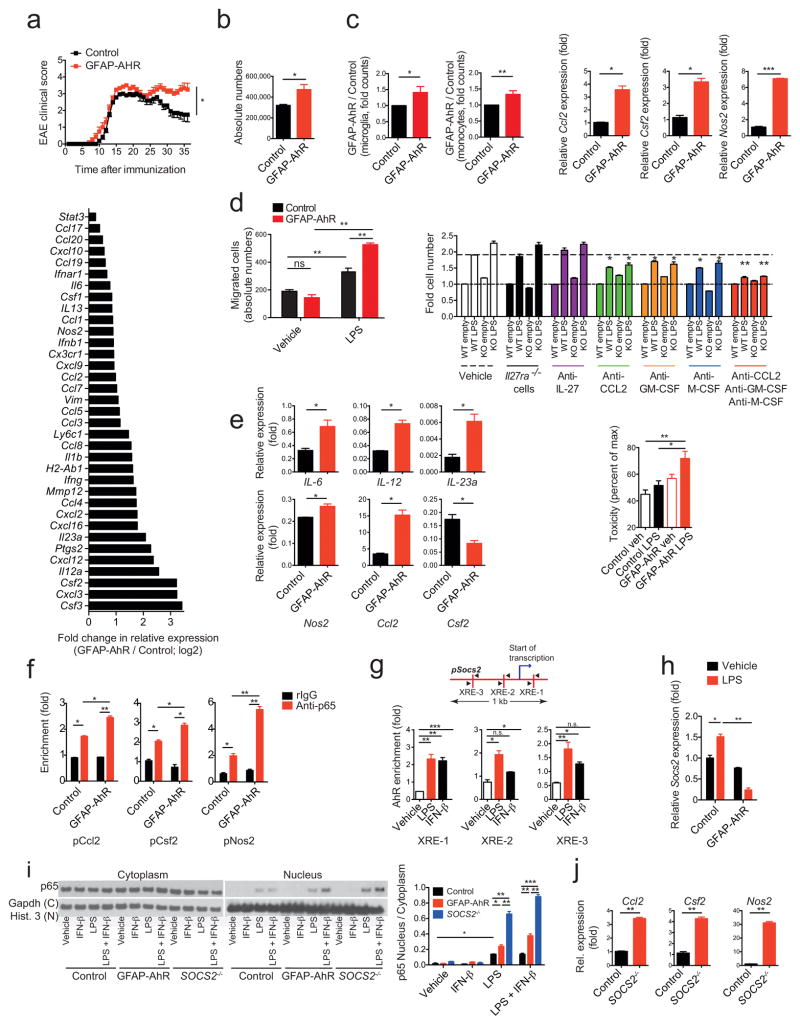
Figure 4. AhR in astrocytes limits CNS inflammation.
EAE in GFAP-AhR-deficient or control mice. (a) Top: Clinical scores (mean ± s.e.m.; representative out of five independent experiments with n = 10 mice per group; Two-way ANOVA). Bottom: Ratio of RNA abundances in pro-inflammatory cluster from sorted GFAP-AhR-deficient and control astrocytes at the peak of disease (fold change in relative expression as determined by log2(GFAP-AhR/Control). Representative of two independent experiments of pooled astrocytes of n = 3 mice per group. (b) Absolute number of CNS infiltrating CD11b+Ly-6Chi monocytes as assessed by FACS analysis. n = 5 per group, representative of five independent experiments, Student’s t-test. (c) Left two figures: Nanostring analysis of pro-inflammatory gene clusters (Supplementary Table 3) from sorted CD11b+CD45lo microglia (left) and CD11b+Ly-6Chi monocytes (right); numbers of GFAP-AhR divided by Control; representative out of two independent experiments of pooled microglia and macrophages of n = 3 mice per group; Student’s t-test. Right three figures: RNA expression of indicated genes in astrocytes sorted from WT and GFAP-AhR mice at peak of disease. (n = 3, Student’s t-test; normalized to Control Ccl2) (d) Left: Supernatants of LPS or vehicle stimulated WT or GFAP-AhR-deficient astrocytes were investigated in migration assays using CD11b+Ly6Chi WT monocytes as migrating cells (absolute cell numbers; n = 3; representative of three independent experiments; one-way ANOVA, Tukey’s multiple comparisons test). Right: Migration assay using blocking antibodies as indicated or IL-27R KO macrophages (fold cell numbers; n = 3; representative of three independent experiments; one-way ANOVA within treatment groups, Turkey’s multiple comparisons test). (e) Left panel: Sorted CD11b+Ly6Chi monocytes were co-cultured with activated control or GFAP-AhR-deficient astrocytes, re-isolated and gene-expression analyzed by qPCR (n = 3, representative of two independent experiments; Student’s t-test; normalized to Control Ccl2 in c). Right graph: Neurotoxicity assay with supernatants from control or GFAP-AhR-deficient astrocytes after activation with LPS or vehicle n = 3, representative of two independent experiments, one-way ANOVA, Tukey’s multiple comparisons test). (f) ChIP analysis of binding of NF-kB (p65) to the promoters of Ccl2, Csf2 and Nos2 in Control or GFAP-AhR-deficient astrocytes after activation with LPS. (n = 3, representative of two independent experiments, one-way ANOVA, Tukey’s multiple comparisons test). (g) Schematic of predicted AhR binding sites (XREs) in the SOCS2 promoter (upper graph) and ChIP analysis of AhR binding to the promoter of SOCS2 in astrocytes after stimulation with indicated conditions (lower bar graphs). (n = 3, representative of two independent experiments, one-way ANOVA, Tukey’s multiple comparisons test; f, g normalized to pCcl2 Control rIgG). (h) Relative expression of Socs2 in WT or GFAP-AhR astrocytes after stimulation with LPS (representative out of two independent experiments; one-way ANOVA, Tukey’s multiple comparisons test; normalized to Control Vehicle). (i) Western blot detecting NF-kB (p65; left) and quantification (right) of the ratio of nuclear to cytoplasmatic fraction of WT, GFAP-AhR, and SOCS2−/− astrocytes stimulated with indicated conditions (representative out of three independent experiments, one-way ANOVA, Tukey’s multiple comparisons test). (j) qPCR of expression levels of Ccl2, Csf2, and Nos2 in Control and SOCS2−/− astrocytes after stimulation with LPS (representative out of two independent experiments; Student’s t-test; normalized to Control Ccl2). Significance levels: * P<0.05, ** P<0.01, *** P<0.001.