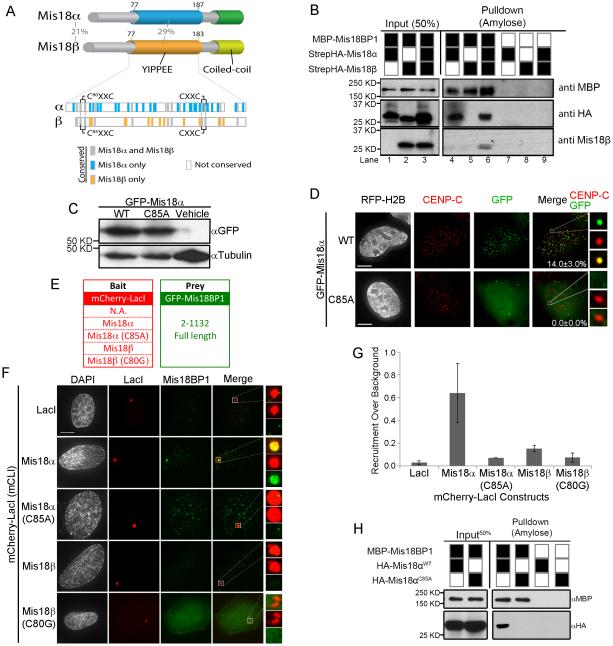
Figure 3.
Mis18α interacts with Mis18BP1 through conserved cysteine residues. (A) Domain structure of human Mis18α and Mis18β paralogs. The percent amino acid identity between the human Mis18α and Mis18β paralogs is shown for the entire protein and within the YIPPEE domain. Amino acids within the YIPPEE domain conserved across all vertebrate Mis18 genes (gray), only in Mis18α (blue) or only in Mis18β (orange) are shown. (B) Amylose bead pull downs of recombinant MBP-Mis18BP1 incubated with StrepHA-Mis18α and StrepHA-Mis18β. (C) Immunoblot of GFP-Mis18α wild type or the C85A mutant in expressed in U2OS cells. (D) Localization of either GFP-Mis18α wild type and C85A mutant to centromeres The percentage of cells with centromeric GFP signal ± standard deviation. Scale bar, 5 μm. (E) mCLI bait constructs and the GFP-Mis18BP1 prey constructs. (F) mCLI-Mis18α or -Mis18β constructs targeted to the LacO array and the recruitment of full length GFP-Mis18BP1. (G) Quantitation of GFP-Mis18BP1 recruitment to the LacO array, ± S.D (H) Amylose pull downs of recombinant MBP-Mis18BP1 incubated with HA-tagged wild type Mis18α or the C85A. See also Figure S2-S4.