Figure 7.
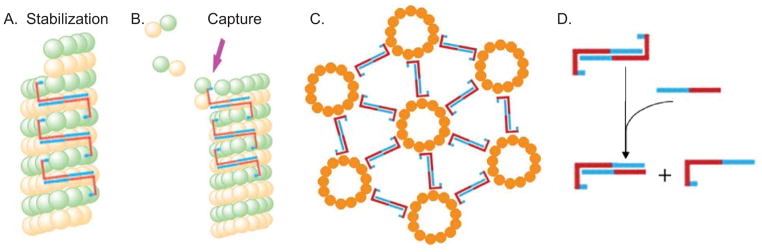
Possible models for how tau dimerization might promote normal and pathological tau action. In all panels, blue corresponds to the acidic regions of tau while red corresponds to basic regions of tau. Panels A and B suggest how tau anti-parallel dimerization could suppress MT shortening and promote MT growth events, respectively. (A) The two MT binding domains within a tau dimer bind to two different tubulin dimers (or two different small groups of neighboring dimers) within a single MT while the dimerized projection/zipper domain extends over a number of intervening tubulin dimers. This locks the bound tubulin subunits into place. The tubulin subunits at the extreme end of the MT are not tau bound and therefore have a higher probability of dissociating. However, upon dissociating to the position at which tubulin subunits are bound by a tau dimer, there would be a much lower probability of further tubulin dissociation and therefore MT shortening events would be suppressed and MT rescues promoted. (B) One MT binding domain of a tau dimer is bound to a tubulin dimer near the end of the MT while the other MT binding domain of the dimer extends out into the cytoplasm. The free end of the tau dimer could now bind/capture a free tubulin dimer in the cytoplasm, thereby increasing the probability of that tubulin dimer integrating into the MT lattice at the growing end. This mechanism would promote MT growth events. (C) Panel C depicts how anti-parallel tau dimers could promote MT bundling. One MT binding domain of a tau dimer associates with one MT and the other MT binding domain of the same tau dimer associates with a second MT, leading to a regular, defined distance between bundled MTs. The schematic presents MTs in linear and hexagonal patterns, consistent with the images in Knops et al., (1991). Panel C is based on a figure from Rosenberg et al., 2008. (D) Schematic of full-length tau:tau dimers and possible full-length tau:tau N-terminal region heterodimers, the later of which might serve as competitive inhibitors of normal tau action.