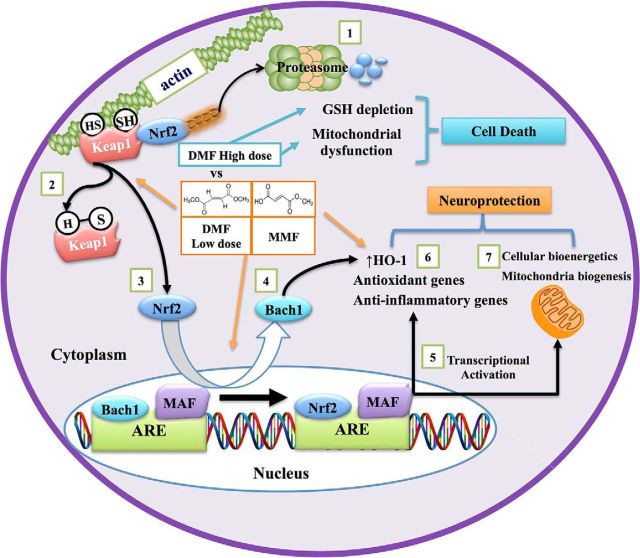
Figure 14.
Mechanism of Nrf2 activation by DMF and MMF and its role in neuroprotection. DMF and MMF differentially activate the Nrf2/ARE pathway to modulate the antioxidant, anti-inflammatory, and mitochondrial biosynthetic machinery. 1, Under basal conditions, Nrf2 is sequestered in the cytoplasm through its binding to its cytoplasmic inhibitor Keap1, which promotes Nrf2 degradation via a ubiquitin proteasome pathway. 2, In the presence of electrophilic agents such as DMF and MMF, the cysteine residues on Keap1 are modified as a result of an alkylation or reduction process, which in turns prevents the ubiquitin-dependent proteasomal degradation of Nrf2. Both DMF and MMF in our study have been shown to possess strong alkylation properties toward thiol groups present on GSH; this characteristic might explain their ability to disrupt the Keap1–Nrf2 interaction by alkylating the cysteine residues on Keap1, leading to nuclear translocation of Nrf2. 3, 4, The Nrf2 pathway is kept in check normally by Bach1, which interacts with small Maf proteins in the nucleus and acts as a repressor of Nrf2-induced ARE-containing gene activation. Once inside the nucleus, Nrf2 binds to the ARE sites of the ARE-containing genes after the export of Bach1 from the nucleus to the cytoplasm. 5, After the export of Bach1 from the nucleus, Nrf2 complexes with small Maf proteins, induces ARE activation, and upregulates a battery of genes involved in antioxidant and anti-inflammatory responses. 6, Both DMF and MMF demonstrate activation of the Nrf2 pathway, although differentially. 7, In our study, we also found that DMF and MMF mediated mitochondrial biogenesis in an Nrf2-dependent manner via an unknown mechanism, accompanied by enhanced cellular bioenergetics to render neuroprotection. Nrf2 activation by DMF, but not MMF, was associated with depletion of GSH, decreased cell viability, and inhibition of mitochondrial oxygen consumption and glycolysis rates in a dose-dependent manner, whereas MMF increased these activities in vitro.