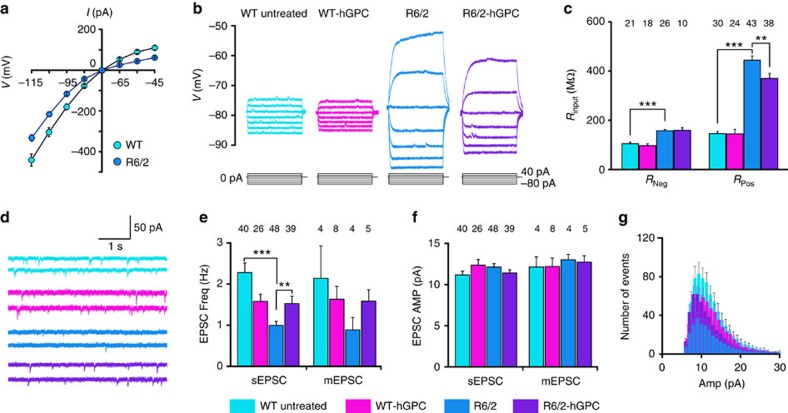
Figure 7. Chimerization with normal glia partially normalizes MSN physiological function.
(a) The current–voltage relationship (I–V) derived from whole-cell V-clamp recordings in WT and R6/2 × rag1−/− (R6/2) mice. (b) Representative whole-cell I-clamp recordings from rag1−/− WT, CD44 hGPC-engrafted rag1−/− WT's, R6/2 × rag1−/− mice and CD44-engrafted rag1−/− mice. Lines below each group of traces indicate the current injection steps. (c) The input resistance Rinput was significantly higher in R6/2 × rag1−/− striatal neurons than in WT × rag1−/− controls, but was partially restored to normal in R6/2 mice chimerized with normal CD44-sorted hGPCs. (d) Representative traces of sEPSCs from striatal neurons recorded in rag1−/− control (black), CD44-engrafted rag1−/− (yellow), R6/2 × rag1−/− (purple) and CD44-engrafted R6/2 × rag1−/− (green) mice. (e,f) The frequency (e) and amplitude (f) of sEPSCs and miniature EPSCs (mEPSCs). (e) The sEPSC frequency was significantly lower in R6/2 striatal neurons than in WT rag1−/− controls, but was restored in CD44-engrafted R6/2s to levels not significantly different from control. (f) In contrast, the EPSP amplitude of R/2 striatal neurons was unaffected by chimerization. (g) Cumulative distribution of sEPSCs. The lower frequency of sEPSCs in the R6/2 MSNs, and partial restoration by hGPC engraftment, was consistent across EPSC amplitudes. WT-untreated, n=11; WT-hGPC, n=8; R6/2-untreated, n=11; R6/2-hGPC, n=8. Means±s.e.m.; *, ** and *** indicates P<0.05, 0.01 and 0.001, respectively, by one-way ANOVA with post hoc t-tests.