Fig. 1.
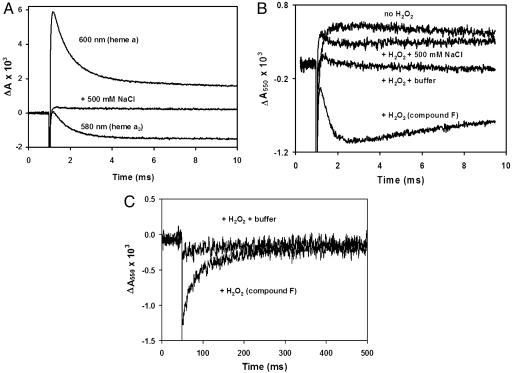
Kinetics of photoreduction of compound F in monomeric CcO. (A) Reduction of compound F in monomeric CcO. Two preparations of bovine CcO were kindly donated by S. Yoshikawa (Japan Science and Technology Corporation, Hyogo, Japan) and O. Einarsdóttir (University of California, Santa Cruz) and showed the same behavior. Monomeric CcO was prepared by four sequential cycles of dilution into 10 mM 2-(N-cyclohexylamino)ethanesulfonic acid, pH 10.1/0.1% dodecylmaltoside, followed by concentration in Amicon concentrators (50-kDa cutoff). The resulting monomeric CcO was reduced overnight with 2 mM ascorbate and 10 μM N,N,N′,N′-tetramethyl-p-phenylenediamine to improve the yield of compound F. After the overnight incubation, CcO was exchanged four times into 100 μM phenolphthalein, pH 9.5, by using the Amicon concentrators. The concentration of CcO was measured by using the absorbance in Soret band as described in ref. 24. The pH of the sample was adjusted to 9.5 by titration with dilute KOH by using the absorbance of the dye at 550 nm. The photoreduction of CcO was achieved as described in ref. 24 by a laser pulse in the presence of 30 μMRu2C, 10 mM aniline, and 1 mM 3CP (the sacrificial electron donor). Compound F was made by incubation with 6 mM H2O2 for 5 min followed by titration of the sample to pH 9.5. After recording the transients, 500 mM NaCl was added to dissociate Ru2C from CcO, and the photolysis was repeated. (B) Proton release upon reduction of compound F. Optical changes were measured in the sample prepared as described for A. The absorbance at 550 nm because of phenolphthalein was recorded in the presence of aniline/3CP before and after H2O2 was added. The small increase in the optical density in the absence of H2O2 is an artifact, which remains after addition of 500 mM NaCl. In the presence of H2O2 the decrease in the dye absorbance is observed concomitant with the reduction of compound F, indicating acidification of the solution. Addition of a known amount of acid (data not shown) was used to calibrate the decrease, which corresponds to 0.8 H+ per molecule of compound F reduced. Measurements with different enzyme samples yielded values ranging from 0.5 to 0.8 H+ per electron. The decrease in dye absorbance was not observed in the presence of buffer. (C) Slow proton uptake after reduction of compound F. The proton uptake is delayed in the monomeric CcO. The absorbance at 550 nm recovers the initial level long after compound F is reduced to the level observed in the presence of the buffer.