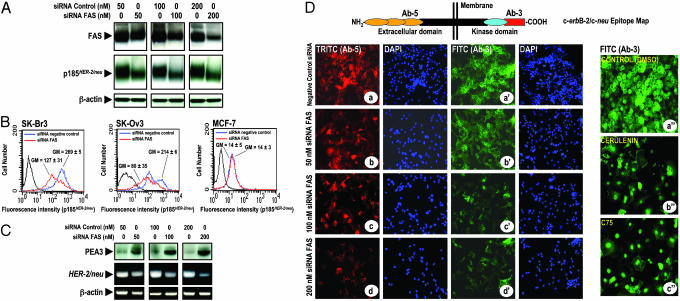
Fig. 3.
(A–C) RNAi-mediated silencing of the FAS gene suppresses HER2 expression. (A) SK-Br3 cells were transfected with siRNA targeting FAS gene or with a nonspecific control siRNA pool for 72 h. Twenty micrograms of protein was subjected to Western blot analyses with specific Abs against FAS, p185HER2, or β-actin. (B) The amount of p185HER2 in FAS RNAi-transfected cells was quantified by flow cytometry using the p185HER2 Ab-5. The mean fluorescence signal ± SD (n = 3) was quantified by using the Geo Mean fluorescence parameter provided with cellquest software. (C)(Upper) Fifty micrograms of protein from FAS RNAi-transfected SK-Br3 cells was subjected to Western blot analyses for PEA3. (Lower) Total RNA from FAS RNAi-transfected SK-Br3 cells was isolated and RT-PCR analyses for HER2 and β-actin transcripts and expression were performed as described in Materials and Methods. (D) Impact of FAS RNAi and pharmacological blockade of FAS activity on p185HER2 cellular localization. Nonspecific RNAi- and FAS RNAi-transfected cells were fixed and labeled with the p185HER2 Ab-5 (a–d) or the p185HER2 Ab-3 (a′–d′). SK-Br3 cells were treated for 72 h with DMSO vol/vol (a″), 2.5 μg/ml cerulenin (b″), or 2.5 μg/ml C75 (c″), and then fixed and labeled with the p185HER2 Ab-3. Cellular localization of p185HER2 was detected by indirect immunofluorescence by incubating with TRITC (a–d)- or FITC (a′–d′)-conjugated anti-mouse IgG. After counterstaining with 4′,6-diamidino-2-phenylindole, cells were examined and photographed by using a Zeiss fluorescent microscope. A representative immunostaining analysis (n = 3) is shown.