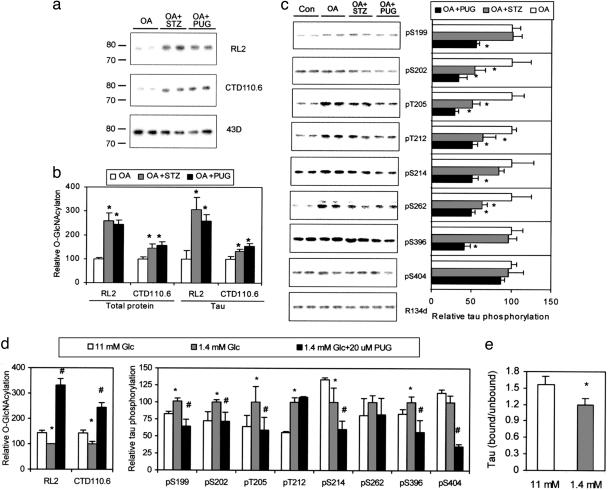
Fig. 2.
Regulation of tau phosphorylation by O-GlcNAcylation in differentiated PC12 cells that stably expressed human tau441.(a) Tau was first immunoaffinity-purified by using phospho-independent tau antibody 43D from PC12 cells treated with OA alone or in combination with either STZ or PUGNAc and then examined by using Western blots developed with O-GlcNAc-specific antibodies RL2 and CTD110.6 or with 43D. (b) The O-GlcNAcylation levels of lysate proteins and immunoaffinity-purified tau were quantitated by 125I-Western blots developed with RL2 and CTD110.6. *, P < 0.05 versus OA group. (c) (Left) The level and phosphorylation state of tau were analyzed by 125I-Western blots developed with phosphorylation-independent tau antibody R134d and phosphorylation-dependent and site-specific tau antibodies, respectively, as indicated. (Right) Quantitations of these blots after normalization with the R134d blot are shown. Mean ± SD of three to four separate experiments is shown. *, P < 0.05 versus OA group. (d) PC12 cells were cultured in medium containing normal (11 mM) or low (1.4 mM) concentration of Glc with or without PUGNAc for 2 days, and then the levels of protein O-GlcNAcylation and tau phosphorylation of the lysates were determined by 125I-Western blots. (e) The microtubule-bound tau and -unbound tau were quantitated by 125I-Western blots. Data in d are the quantitations of the blots after normalization with R134d blot. Mean ± SD of three to four separate experiments is shown in d and e. *, P < 0.05 versus 11 mM Glc group; #, P < 0.05 versus 1.4 mM Glc group.