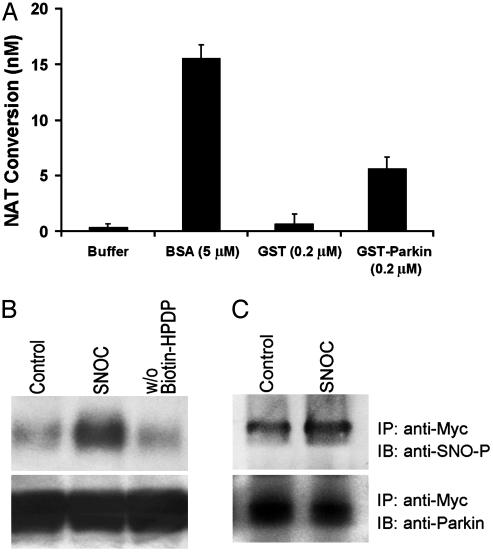
Fig. 1.
S-nitrosylation of parkin in vitro. (A) Recombinant protein GST-parkin (0.2μM) was incubated with SNOC (200μM) for 30 min at room temperature (RT). The SNO-PARK thus generated was assessed by release of NO, causing the conversion of 2,3-diaminonaphthalene to the fluorescent compound NAT. GST protein alone was used as negative control. BSA (5 μM) was used as positive control (25). SNOC itself quickly decayed and thus resulted in insignificant S-nitrosothiol readings in this assay. (B)(Upper) Cell lysates from human neuroblastoma SH-SY5Y cells overexpressing myc-tagged parkin were incubated with 200 μM SNOC at RT. Control samples were subjected to decayed SNOC under the same conditions. Thirty minutes after SNOC exposure, SNO-PARK was detected by the biotin-switch assay (15). w/o Biotin-HPDP represents the control without biotin linker [N-[6-biotinamido)hexyl]-1′-(2′pyridyldithio) propionamide] (Lower) Total parkin in cell lysates identified by immunoblot. (C) (Upper) Cell lysates were immunoprecipitated with anti-myc antibody. The immunoprecipitates were incubated with or without SNOC and subjected to SDS/PAGE Western analysis under nonreducing conditions. The membrane was blotted with anti-S-nitrosylated protein antibody to identify nitrosylated parkin and revealed an ≈2-fold increase after SNOC exposure by densitometry. (Lower) Same blot reprobed with anti-parkin antibody to verify equal loading.