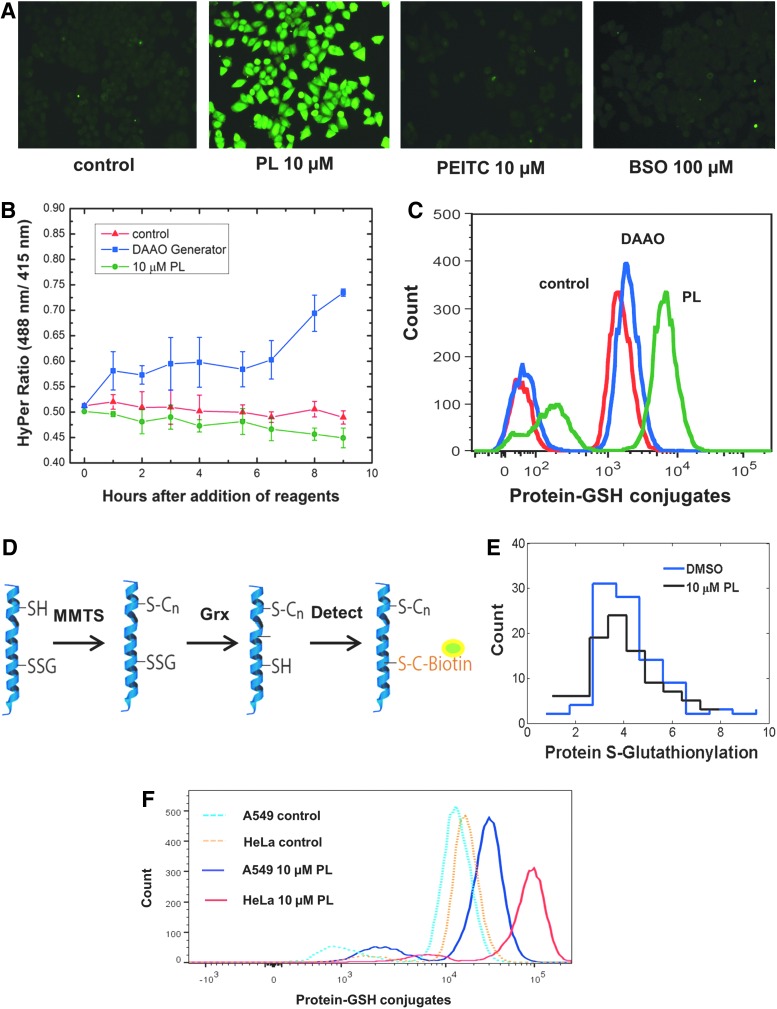
FIG. 6.
Protein-GSH detection as evidence of oxidative stress in response to PL and PEITC. (A) Immunofluorescence detection of protein-GSH conjugate. HeLa cells were incubated for 10 h with 10 μM PL, 10 μM PEITC, and 100 μM BSO, and then, protein-GSH conjugates were detected using an antibody raised against GSH. Only treatment with PL resulted in an increase in the level of protein-GSH adducts. The experiment was repeated two times. (B) HyPer measurement of HeLa cells treated with 10 μM PL and HeLa cells virally transfected with DAAO generator with D-ala substrate added. Error bars represent 95% confidence interval of four technical repeats. (C) Flow cytometry quantification of protein-GSH conjugates for the PL-treated and DAAO-transfected samples in (B). (D) Schematic of an assay to detect mixed disulfides of protein-GSH conjugates. The free thiol groups are blocked using MMTS, and then, the mixed disulfide formed by the oxidized protein and GSH is reduced using Grx. These sulfhydryl groups are detected using biotinylated maleimide. (E) Detection of mixed disulfides in protein-GSH adducts for HeLa cells treated with PL. PL treatment did not cause a rise in free sulfhydryl level after reduction via Grx. The experiment was repeated two times. (F) Flow cytometry quantification of protein-GSH adducts for A549 and HeLa cells treated with 10 μM PL. HeLa cells show much greater increase in protein-GSH complexes compared to the A549 cells. The experiment was repeated two times. Grx, glutaredoxin; MMTS, methyl methanethiosulfonate. To see this illustration in color, the reader is referred to the web version of this article at www.liebertonline.com/ars