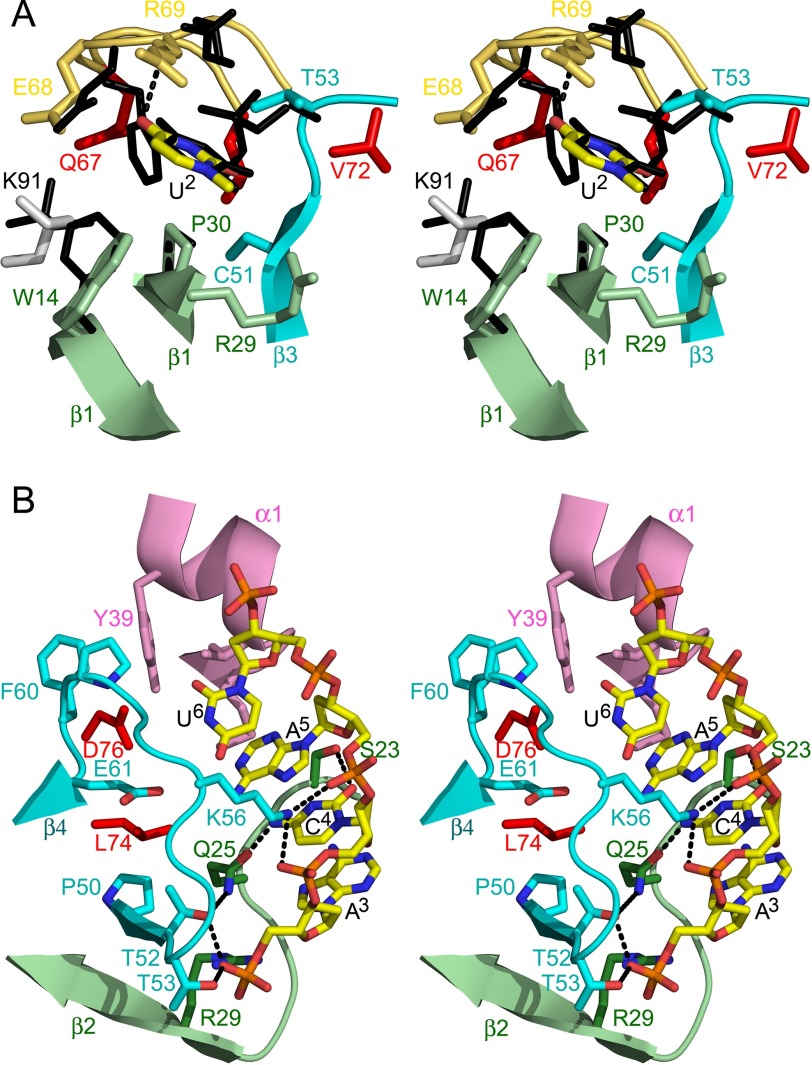
FIGURE 3.
Subsite details. A, details of the interactions in the upstream U binding cleft. Residues of EcMazF interacting with the base are shown in stick representation and colored as in Fig. 1A. The uridine base is surrounded by the aliphatic/aromatic parts of the side chains of Trp-14, Arg-29, Pro-30, Thr-52, Thr-53, and Arg-69. The specificity-determining hydrogen bond from the main chain NH of Arg-69 to O4 of the uridine base is shown as a gray dotted line. Amino acid side chains that are part of the bottom of the binding site but do not touch the substrate are colored red. The equivalent residues of BsMazF are superimposed as black sticks and labeled in black. B, details of the interactions in the downstream ACA-binding groove. Residues of EcMazF interacting with d(A3C4A5U6) are shown in stick representation and colored as in Fig. 1A. Corresponding secondary structure elements are shown as a schematic. Hydrogen bonds are represented as dotted lines. Amino acid side chains that are part of the bottom of the binding groove but do not touch the substrate are colored red.