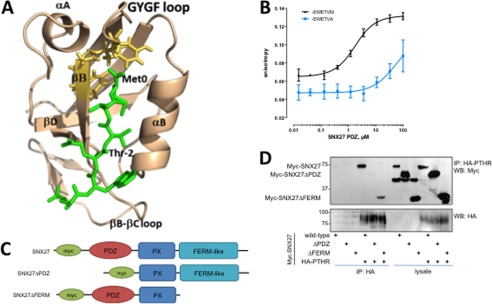
FIGURE 3.
Structural modeling and measurement of SNX27 binding to PTHR. A, model structure of the SNX27 PDZ domain in complex with the C-terminal PTHR peptide. The PDZ domain and PTHR peptide are shown in wheat and green, respectively. The PTHR peptide (-EWETVM) is shown in a stick representation with residues numbered from 0 to −5. The GYGF loop of the PDZ domain is shown in yellow (stick representation). Residues forming the canonical hydrophobic pocket of PDZ are shown in wheat (stick representation). B, fluorescence anisotropy of SNX27 PDZ domain binding to PTHR. Representative binding curves for the FITC-labeled -EWETVM and -EWETVA peptides to the SNX27 PDZ domain are shown (n = 3). C, schematic representation of full-length Myc-tagged SNX27, PDZ deletion (SNX27ΔPDZ), and FERM deletion (SNX27ΔFERM) constructs used here. D, coimmunoprecipitation characterization of SNX27 structural determinants for PTHR binding. HEK293 and HA-PTHR stable cells were transiently transfected with the indicated Myc-SNX27 construct. Cells were treated with 100 nm PTH (5 min) and lysed in modified RIPA buffer. HA-PTHR was immunoprecipitated with anti-HA-conjugated Sepharose beads. The presence of bound SNX27 was detected by immunoblotting. Images are representative of n = 3 independent experiments. The results establish that PTHR binds to SNX27 by virtue of its PDZ domain. Error bars, S.E.