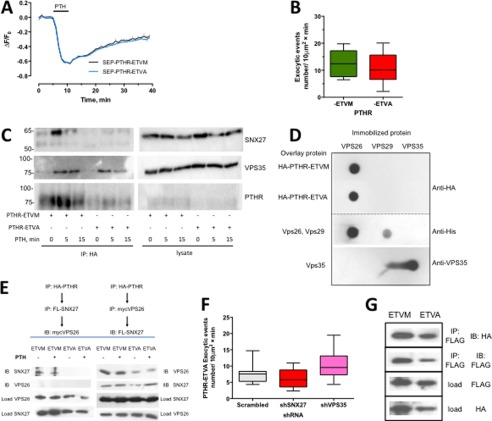
FIGURE 6.
PDZ ligand autonomous PTHR-retromer interaction and recycling. A, whole cell recycling of PTHR with intact or disrupted PDZ ligand measured by time lapse confocal microscopy. Recycling of either intact SEP-PTHR-ETVM (black) or mutant SEP-PTHR-ETVA (blue) was measured following PTH-induced receptor internalization. After a 5-min baseline was established, cells were treated with 100 nm PTH for 5 min and then washed out (n = 10, ETVM, ETVA). B, single exocytic events were measured by time lapse TIRF microscopy as before. At 5 min after the addition of 100 nm PTH, the rate of exocytosis was indistinguishable between PTHR-ETVM (12.8 ± 1.9 exocytic events) and PTHR-ETVA (11.3 ± 1.7 exocytic events) (p = 0.62). C, coimmunoprecipitation analysis of PTHR-ETVM and PTHR-ETVA binding to SNX27 and VPS35. GnTI HEK293 cells stably expressing HA-PTHR-ETVM or HA-PTHR-ETVA were treated with 100 nm PTH for 5 or 15 min and then cross-linked by incubation in DSP for 2 h on ice. The cross-linking reaction was terminated by the addition of 20 mm Tris (pH 7.5), and the cells were lysed in RIPA buffer. HA-PTHR was immunoprecipitated using anti-HA-conjugated Sepharose beads, and the presence of SNX27 and VPS35 in complex with the receptor was determined by immunoblotting. SNX27 and VPS35 were present in abundance after a 5-min PTH treatment of HA-PTHR-ETVM cells and at a lesser degree after 15 min. SNX27 did not coimmunoprecipitate with PTHR-ETVA, but binding to retromer could be detected at both 5 and 15 min after PTH treatment. D, overlay analysis of PTHR-ETVA and PTHR-ETVA interactions with purified VPS protomers. VPS26, VPS29, and VPS35 protein were applied to Immobilon P membranes under vacuum. 10 μg of HA-PTHR-ETVA or HA-PTHR-ETVA protein, as indicated, was overlaid. HA-PTHR bound to the retromer components was detected with a polyclonal anti-HA antibody. The presence of immobilized His-VPS26 and His-VPS29 was confirmed with a monoclonal anti-His antibody; immobilized VPS35 was established with a polyclonal anti-VPS35 antibody. Images are representative of three independent experiments. E, sequential assembly of a PTHR-VPS26-SNX27 protein complex. The top panel shows the sequence in which the serial immunoprecipitation was performed, and the bottom panel shows the respective results. The PTHR forms a ternary complex only when it first interacts in VPS26. The results are illustrative of four independent experiments. F, comparison of SNX27 and VPS35 down-regulation on PTHR-ETVA recycling. HEK293 cells stably expressing SEP-PTHR-ETVA were transiently transfected for 72 h with shRNA against SNX27 or VPS35. Exocytosis was measured by time lapse TIRF microscopy. Activity-dependent recycling of the PTHR was not altered by shRNA knockdown of VPS35 or SNX27 compared with control shRNA (scrambled shRNA, 7.6 ± 0.8 (n = 11); shSNX27, 6.123 ± 0.8 (n = 11); shVPS35, 10.1 ± 1.0 (n = 15) exocytic events). G, NSF binding to PTHR-ETVM and PTHR-ETVA. CHO cells were transfected with FLAG-PTHR-ETVM or -ETVA, as indicated, and HA-NSF. PTHR was immunoprecipitated with FLAG-M2 affinity gel. PTHR and NSF were detected by immunoblotting using anti-FLAG and anti-HA antibodies, respectively, as described under “Experimental Procedures.” Similar results were obtained in four independent experiments. Error bars, S.E.