FIGURE 8.
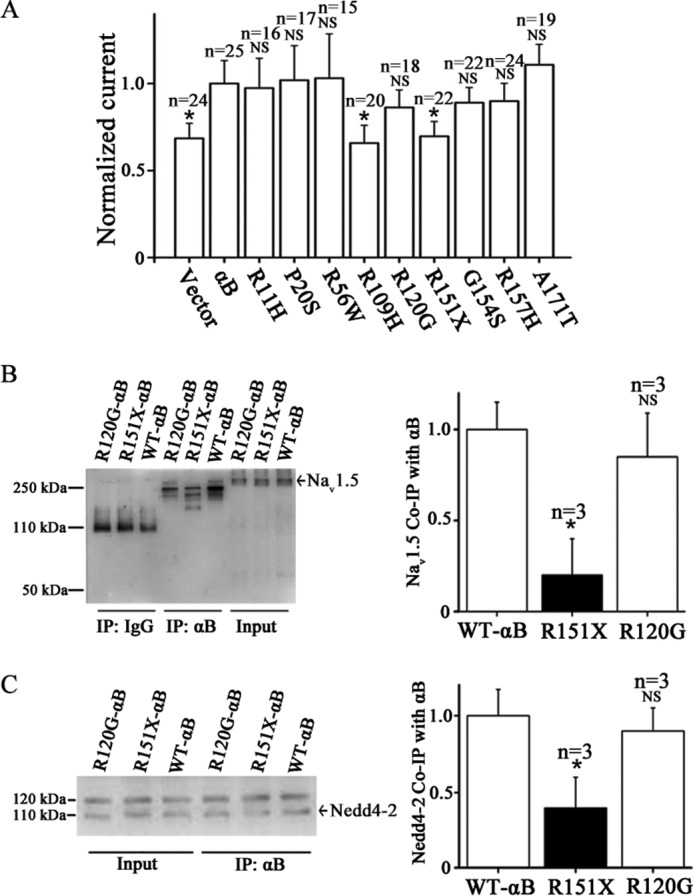
Functional effects of αB-crystallin mutations associated with human diseases on peak INa densities and the interaction of αB-crystallin with either Nav1.5 or Nedd4-2. A, HEK/Nav1.5 cells were transfected with an expression plasmid for wild type αB-crystallin or mutant αB-crystallin and then used for whole-cell patch clamp recordings of INa. The peak sodium current density was normalized to the sodium current density recorded from HEK/Nav1.5 cells transfected with wild type αB-crystallin and plotted. B, co-IP analysis for interaction between wild type αB-crystallin or two mutant αB-crystallin proteins and Nav1.5. C, co-IP analysis for interaction between wild type αB-crystallin or two mutant αB-crystallin proteins and Nedd4-2. NS, not significant; *, significant with p < 0.05. Data are shown as means ± S.E. (error bars) All studies were repeated at least three times.
