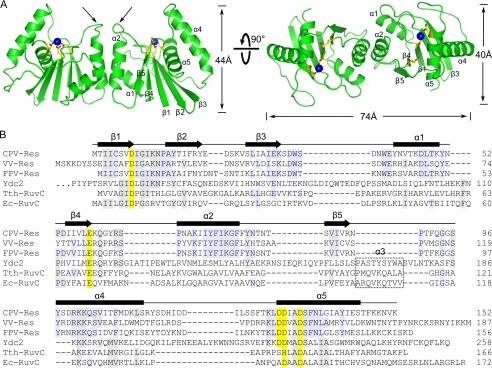
FIGURE 2.
Overall structure of CPV resolvase. A, orthogonal views of the resolvase dimer. The active sites are indicated by select side chains (yellow/red) and bound Mg2+ (blue spheres). Dimerization is mediated primarily by the α2 helix. Arrows indicate the β4-α2 loop, which adopts different conformations in the two subunits. B, alignment of RuvC family resolvases. Secondary structure is indicated for CPV resolvase. Active site residues are shaded yellow, and residues identical in three or more of the sequences are shaded gray. The α3 helix present in bacterial and mitochondrial resolvases is boxed. Ydc2, Schizosaccharomyces pombe; Tth, T. thermophilus; Ec, E. coli.