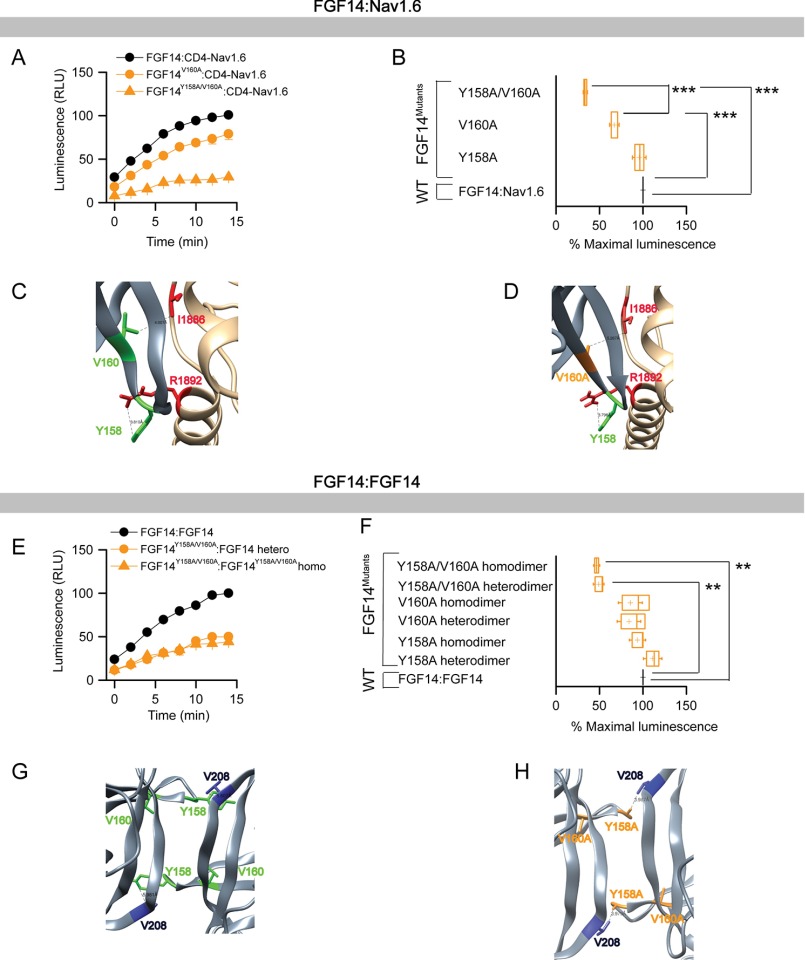
FIGURE 4.
Differential role of Tyr-158 and Val-160 at the FGF14·Nav1.6 channel and the FGF14:FGF14 dimer interface assessed by alanine-scanning mutagenesis and in-cell LCA. A, representative luminescence response (RLU) corresponding to the assembly of CLuc-FGF14·CD4-Nav1.6-NLuc (black circle) and respective mutants (V160A, orange circle; Y158A/V160A, orange triangle). B, box plot representing percent maximal luminescence response of relative mutants normalized to the CLuc-FGF14· CD4-Nav1.6-NLuc control (black). C, homology model of the FGF14·Nav1.6 complex (zoom view) in which FGF14 is shown as gray and C-tail of Nav1.6 is shown as tan. Tyr-158 (green) and Val-160 (green) interact respectively with Arg-1892 and Ile-1886 of C-tail of Nav1.6. D, interaction between V160A (orange) of FGF14 with Ile-1886 (red) of C-tail of Nav1.6 is shown in the FGF14V160A·Nav1.6 homology model. E, representative luminescence response (RLU) corresponding to the assembly of CLuc-FGF14·FGF14-NLuc (black) and the respective mutants (Y158A/V160A heterodimer, orange circle; Y158A/V160A homodimer, orange triangle). F, box plot representing percent maximal luminescence response of relative mutants normalized to the CLuc-FGF14:FGF14-NLuc (black) homodimer response. Data are mean ± S.E. The statistical significance between the three groups was assessed using Kruskal-Wallis one-way ANOVA on ranks with post hoc Dunn's method; ***, p < 0.001; **, p < 0.01. G, homology model of the FGF14:FGF14 homodimer (zoom view) in which Tyr-158 (green) from one FGF14 monomer interacts with Val-208 of the neighboring FGF14. H, interaction between Y158A (orange) of FGF14 with Val-208 (blue) of the neighboring FGF14 monomer is shown in the FGF14Y158A/V160A:FGF14Y158A/V160A homodimer model.