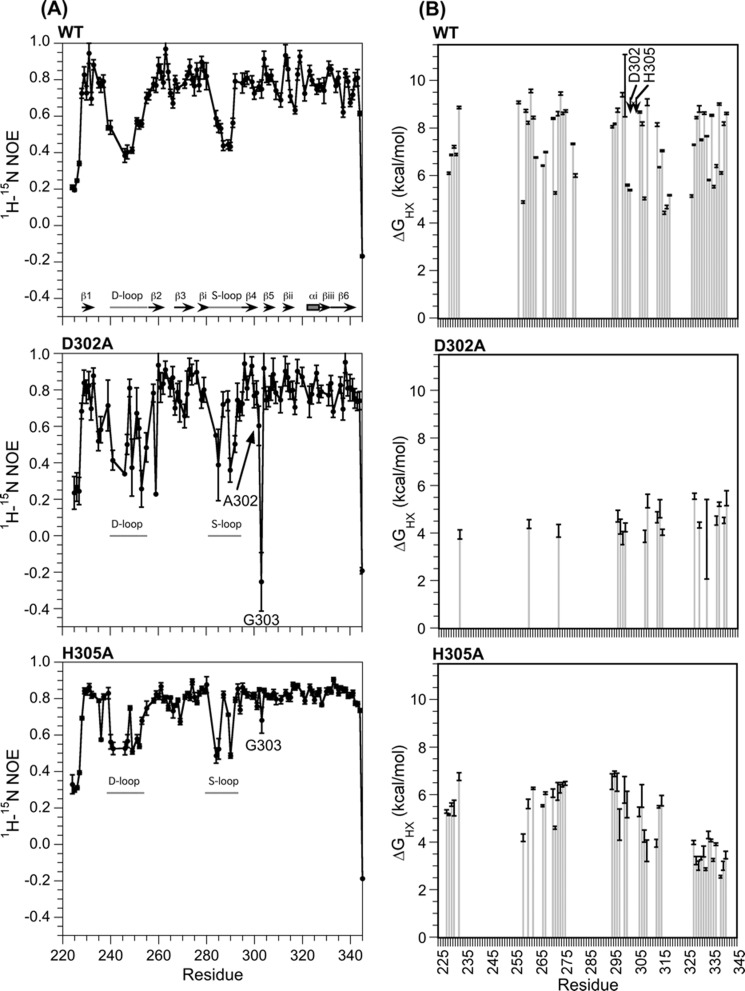
FIGURE 4.
Effects of mutations on I-domain dynamics. A, 1H,15N NOE values monitoring backbone dynamics on the ps-ns timescale. All 1H,15N NOE data were obtained at 20 °C. Because the least stable mutant D302A is prone to aggregation, data were collected using a 0.1 mm sample of D302A compared with 0.5 mm for WT and H305A. The lower protein concentration for D302A decreased NMR sensitivity; consequently the 1H,15N NOE data for D302A have larger experimental uncertainties. B, hydrogen exchange protection, expressed as ΔGHX values (Equation 2). All hydrogen exchange experiments were done at a pD of 6.0 and a temperature of 25 °C.