Abstract
Background:
Numerous studies have reported predictors of new-onset postoperative atrial fibrillation (POAF) following cardiac surgery, which is associated with increased length of stay, cost of care, morbidity, and mortality. The purpose of this study was to examine the association between preoperative diastolic function and occurrence of new-onset POAF in patients undergoing a variety of cardiac surgeries at a single institution.
Methods:
Using data from a prospective study from November 2007 to January 2010, a retrospective review was conducted. The diastolic function of each patient was determined from preoperative transthoracic echocardiograms. Occurrence of new-onset POAF was prospectively noted for each patient in the original study. Demographic and operative characteristics of the study population were analyzed to determine predictors of POAF.
Results:
Of 223 patients, 91 (40.8%) experienced new-onset POAF. Univariate predictors of POAF included increasing age, male gender, operations involving mitral valve repair/replacement, nonsmoking, hypertension, increased intraoperative pulmonary artery pressure, grade I diastolic dysfunction, abnormal diastolic function of any grade, decreased medial e’, elevated medial E/e’, and increased left atrial volume. Multivariate predictors of POAF included increasing age, increased left atrial volume, and elevated initial intraoperative pulmonary artery pressure. Even after exclusion of patients with hypertrophic obstructive cardiomyopathy or those undergoing mitral valve operations, diastolic dysfunction was not a multivariate predictor of POAF.
Conclusions:
In the patient population studied here, preoperative diastolic dysfunction was not predictive of POAF. In addition to increasing age, initial intraoperative pulmonary artery systolic pressure and left atrial volume were both significant multivariate predictors of POAF.
Keywords: Atrial fibrillation, Cardiac surgery, Diastolic dysfunction, Diastolic function, Postoperative
INTRODUCTION
Postoperative atrial fibrillation (POAF) affects 18–64% of patients following cardiac surgery.[1,2,3,4,5,6,7,8,9,10,11,12] While controversy exists as to whether POAF increases morbidity and mortality or is merely associated with it,[8] at a minimum it leads to an increased length of hospital stay and cost of care.[1,7,11,13,14]
Multiple studies have reported perioperative predictors of POAF.[2,6,7,8,12,14] Predictive models for POAF are of interest as they may allow for selective use of preventative strategies in at-risk patients so that the benefits of therapy outweigh associated risks. A recent study found abnormal preoperative diastolic function to be associated with POAF in patients undergoing coronary artery bypass grafting and/or valvular repair/replacement.[12] To our knowledge, this is the only study to report diastolic dysfunction as a risk factor for POAF.
Given that only one previous study has shown diastolic dysfunction to be associated with POAF, the purpose of the current study was to examine the association between preoperative diastolic function and occurrence of new-onset POAF in patients undergoing a variety of cardiac surgeries at a single institution.
METHODS
This study was approved by the local institutional review board. A retrospective review was conducted from data obtained from a randomized, double-blind, placebo-controlled trial aimed at determining whether or not a single bolus of lidocaine or amiodarone intraoperatively was effective at preventing ventricular fibrillation after aortic cross-clamp removal in patients undergoing cardiac surgery.[15] All patients had provided written informed consent for the original trial acknowledging that further review of their records may occur. Enrollment occurred from November 1, 2007 to January 1, 2010. A blinded study coordinator followed all patients during their hospitalization for the occurrence of new-onset POAF based on electrocardiographic evidence or notations in hospital notes. These data were maintained in a prospective fashion. Patients were included in the current study if they had completed the original trial, were 18 years of age or greater, had undergone resting transthoracic echocardiogram at our institution within 1-year of operation, and had no history of preoperative atrial fibrillation or atrial flutter.
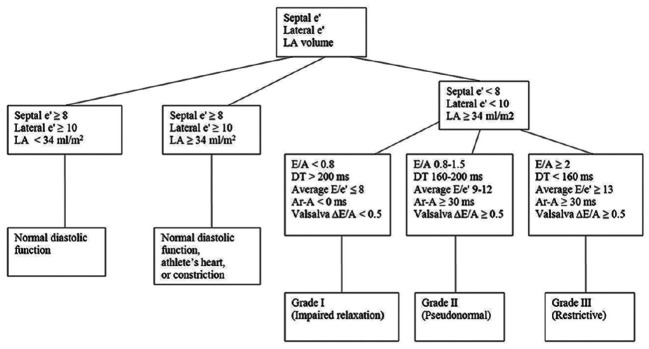
Preoperative, intraoperative, and postoperative data collected during the original study were utilized.[15] Blinded authors (DWB, WJM) retrieved additional echocardiographic data related to diastolic function from the electronic preoperative resting transthoracic echocardiogram report. Two blinded authors (KHR, WJM) certified in advanced perioperative transesophageal echocardiography graded each available patient's diastolic function according to guidelines provided by the American Society of Echocardiography [Figure 1].[16] Discrepancies in grading were resolved by a third blinded author (JNP) certified in advanced perioperative transesophageal echocardiography. Preoperative transesophageal echocardiograms are not routinely performed at our institution for all patients and were, therefore, not included in the determination of diastolic function. In addition, due to rapidly changing hemodynamics intraoperatively, parameters of diastolic function are not typically interrogated intraoperatively with transesophageal echocardiography and were not included in this study.
Figure 1.
Approach to determination of diastolic function. 1Deceleration time, left atrial 1Reproduced with permission from Nagueh et al.[16]
Descriptive statistics for categorical variables are reported as frequency and percentage while continuous variables are reported as mean (standard deviation) or median (range) as appropriate. Categorical variables were compared between patients with and without POAF using Chi-squared test or Fisher's exact test, and continuous variables were compared using two sample t-test or Wilcoxon rank sum test where appropriate. Logistic regression models were used to find the univariate and multivariate predictors of POAF. The multivariable model considered as univariately significant variables (P < 0.05) with model selection using the stepwise method (backward and forward methods resulted in the same model). All statistical tests were two-sided with the alpha level set at 0.05 for statistical significance.
RESULTS
Of 342 patients completing the original trial,[15] 223 patients met enrollment criteria for the current study. Demographic and surgical characteristics stratified according to POAF occurrence for patients meeting inclusion criteria are shown in Table 1. Preoperative transthoracic echocardiographic findings, stratified according to the occurrence of POAF, are listed in Table 2.
Table 1.
Demographic and operative characteristics of the study population according to new-onset postoperative atrial fibrillation occurrence
| No POAF (n=132) | POAF (n=91) | OR | 95% CI | P | |
|---|---|---|---|---|---|
| Age (years) | 57.3±14.8 | 67.4±11.2 | 1.06 | 1.03-1.08 | <0.001 |
| Height (cm) | 172.1±10.2 | 173.5±10.2 | 1.01 | 0.99-1.04 | 0.303 |
| Weight (kg) | 87.4±17.2 | 92.3±20.4 | 1.01 | 1-1.03 | 0.055 |
| Gender: Female | 46 | 20 | 0.53 | 0.29-0.97 | 0.040 |
| Left ventricular ejection fraction (%) | 64.9±9.9 | 63.3±11.8 | 0.99 | 0.96-1.01 | 0.283 |
| Proceduresa | |||||
| CABG performed | 42 | 33 | 1.22 | 0.69-2.14 | 0.490 |
| Aortic valve repair/replacement performed | 38 | 36 | 1.62 | 0.92-2.85 | 0.094 |
| Mitral valve repair/replacement performed | 14 | 19 | 2.22 | 1.05-4.71 | 0.037 |
| Tricuspid valve repair/replacement performed | 1 | 5 | 7.62 | 0.87-66.31 | 0.066 |
| Septal myectomy performed | 59 | 31 | 0.64 | 0.37-1.11 | 0.113 |
| Other procedure (s) performed | 3 | 3 | 1.47 | 0.29-7.43 | 0.644 |
| Medical history | |||||
| Diabetes mellitus | 10 | 14 | 2.22 | 0.94-5.24 | 0.069 |
| Coronary artery disease | 48 | 33 | 1.00 | 0.57-1.74 | 0.988 |
| Current smoker | 15 | 3 | 0.27 | 0.07-0.95 | 0.041 |
| COPD | 4 | 3 | 1.09 | 0.24-5.00 | 0.910 |
| Hypertension | 47 | 48 | 1.05 | 1.17-3.48 | 0.011 |
| Preoperative medications | |||||
| Beta blocker | 83 | 56 | 0.94 | 0.54-1.64 | 0.839 |
| ACE inhibitor/ARB | 29 | 28 | 1.58 | 0.86-2.90 | 0.140 |
| Statin | 51 | 41 | 1.30 | 0.76-2.24 | 0.339 |
| Digoxin | 1 | 2 | 2.94 | 0.26-33.00 | 0.381 |
| Amiodarone | 1 | 1 | 1.46 | 0.09-23.60 | 0.792 |
| Initial mean intraoperative pulmonary artery pressure (mmHg) | 30.5±8.4 | 34.3±10.4 | 1.05 | 1.01-1.08 | 0.005 |
| Duration of aortic cross-clamp (min) | 45.1±26.9 | 52.7±34.3 | 1.01 | 1.00-1.02 | 0.067 |
| Duration of cardiopulmonary bypass (min) | 63.7±39.2 | 73.1±43.3 | 1.01 | 1.00-1.01 | 0.105 |
OR: Odds ratio, CI: Confidence interval, ACE: Angiotensin converting enzyme, ARB: Angiotensin receptor blocker, COPD: Chronic obstructive pulmonary disease, CABG: Coronary artery bypass grafting, POAF: Postoperative atrial fibrillation. aProcedure performed may be in conjunction with other procedures listed as such totals exceed 132 for no POAF and 91 for POAF
Table 2.
Echocardiographic characteristics of the study population according to new-onset postoperative atrial fibrillation occurrence
| Variable | No POAF (n=132) (%) | POAF (n=91) (%) | OR | 95% CI | P |
|---|---|---|---|---|---|
| Left ventricular mass (g) | 272±101 | 294±115 | 1.00 | 1.00-1.00 | 0.147 |
| Left ventricular mass index (g/m2) | 135±45 | 142±52 | 1.00 | 1.00-1.01 | 0.269 |
| Left atrial volume (cc) | 85±28 | 97±41 | 1.01 | 1.00-1.02 | 0.018 |
| E wave velocity (m/s) | 0.871±0.29 | 0.886±0.37 | 1.15 | 0.50-2.61 | 0.746 |
| A wave velocity (m/s) | 0.808±0.29 | 0.867±0.28 | 2.08 | 0.79-5.41 | 0.136 |
| E/A ratio | 1.18±0.53 | 1.11±0.624 | 0.78 | 0.47-1.28 | 0.325 |
| Deceleration time (ms) | 230±57 | 235±61 | 1.02a | 0.97-1.06 | 0.532 |
| e’ medial (m/s) | 0.065±0.06 | 0.054±0.02 | 0.23b | 0.06-0.88 | 0.032 |
| e’ lateral (m/s) | 0.073±0.03 | 0.069±0.03 | 0.51b | 0.16-1.59 | 0.244 |
| E/e’ medial | 15.8±7.3 | 18.0±9.1 | 1.03 | 1.00-1.07 | 0.049 |
| E/e’ lateral | 13.0±6.3 | 13.6±6.6 | 1.01 | 0.97-1.06 | 0.528 |
| Diastolic grade I (impaired relaxation)c | 46 (34.8) | 42 (46.2) | 2.57 | 1.15-5.76 | 0.021 |
| Diastolic grade II (pseudonormal)c | 46 (34.8) | 32 (35.2) | 1.96 | 0.86-4.46 | 0.109 |
| Diastolic grade III (restrictive)c | 9 (6.8) | 6 (6.6) | 1.88 | 0.54-6.50 | 0.319 |
| Any abnormal diastolic gradec | 101 (76.5) | 80 (87.9) | 2.23 | 1.06-4.72 | 0.035 |
POAF: Postoperative atrial fibrillation, CI: Confidence interval, OR: Odds ratio. aOR determined per 10 ms, bOR determined per 0.1 m/s, cOR as compared to normal diastolic function
Ninety-one of the 223 patients (40.8%) experienced POAF. Univariate patient and surgical predictors of POAF included increasing age, male gender, operations involving mitral valve repair/replacement, nonsmoking, hypertension, and increased initial intraoperative pulmonary artery pressure [Table 1]. Mean time from preoperative transthoracic echocardiogram to surgery was 36 ± 47 days (median 22 days). Preoperative echocardiographic predictors of POAF on univariate analysis were grade I diastolic dysfunction, abnormal diastolic function of any grade, decreased medial e’, elevated medial E/e’, and increased left atrial volume [Table 2].
On multivariate analysis, age, increased left atrial volume, and elevated initial intraoperative pulmonary artery pressure were associated with POAF. No individual echocardiographic measurements of diastolic function parameters aside from left atrial volume were associated with POAF on multivariate analysis [Table 3].
Table 3.
Multivariate analysis of predictors of new-onset postoperative atrial fibrillation
| Variable | OR | 95% CI | P |
|---|---|---|---|
| Age (years) | 1.06 | 1.04-1.09 | <0.001 |
| Pulmonary artery systolic pressure (mmHg) | 1.04 | 1.00-1.08 | <0.001 |
| Left atrial volume (cc) | 1.01 | 1.00-1.02 | <0.001 |
CI: Confidence interval, OR: Odds ratio
Due to controversies in diastolic function determination in patients with hypertrophic obstructive cardiomyopathy (HOCM),[16] the data were reanalyzed after exclusion of 92 HOCM patients. In the remaining 131 patients, multivariate predictors of POAF included age (odds ratio [OR]: 1.06, 95% confidence interval [CI]: 1.03–1.11, P < 0.001) and elevated initial intraoperative mean pulmonary artery pressure (OR: 1.04, 95% CI: 1.00–1.08, P = 0.038).
Given that left atrial enlargement and pulmonary hypertension may result from mitral valve dysfunction, the data were reanalyzed after exclusion of 33 patients who underwent mitral valve operation. Of the remaining 190 patients, multivariate predictors of POAF included age (OR: 1.06, 95% CI: 1.03–1.09, P < 0.001), male gender (OR: 2.14, 95% CI: 1.00–4.58, P = 0.0495), and elevated initial intraoperative pulmonary artery pressure (OR: 1.05, 95% CI: 1.01–1.09, P = 0.0195).
Randomization of patients in the original trial had no effect on the incidence of POAF. Of patients experiencing POAF, 29/91 (32%) had received an intraoperative intravenous bolus of amiodarone, compared to 46/132 (35%) who did not experience POAF (P = 0.648).
Eighty of 91 patients (88%) who developed POAF were treated with amiodarone in the postoperative period, compared with 11/132 (8%) without POAF. Patients who developed POAF had longer stays in the intensive care unit (1.2 ± 1.0 days vs. 1.5 ± 1.9 days, P = 0.001) and in the hospital (5.2 ± 1.8 vs. 7.2 ± 4.9, P < 0.001) than did patients who were free of POAF.
DISCUSSION
In the current study, neither individual echocardiographic parameters of diastolic function or grade of diastolic function were predictors of POAF on multivariate analysis. However, pulmonary artery systolic pressure and left atrial volume were both significant predictors of POAF. Thus, clinical sequelae of diastolic dysfunction may be of value in predicting patients that will develop POAF after cardiac surgery.
To our knowledge, only one previous study has evaluated the association between diastolic function and POAF. Melduni et al. reported on 351 patients also from our institution undergoing isolated coronary artery bypass grafting and/or valve repair/replacement.[12] No patients from that study were included in the current study. The rates of POAF were similar between the previous study and ours (38.5% and 40.8% respectively). Grade 0 (normal), I, II, and III diastolic dysfunction were encountered preoperatively in 18.8%. 39.5%, 35.0%, and 6.7%, respectively, of all patients in the current study, compared to 22.5%, 29.6%, 41.1%, and 6.8% in the study of Melduni et al. Left ventricular diastolic function grade was found by Melduni et al. to be an independent predictor for POAF. Conduction heterogeneity secondary to fibrosis of the cardiac chambers associated with aging is hypothesized to increase susceptibility to POAF in the setting of surgical insult.[12,17,18,19] Contrary to Melduni et al., diastolic dysfunction was not associated with POAF in the current study, despite the fact that preoperative diastolic dysfunction of any grade was more common in the current study and both studies had comparable frequencies of pseudonormal (grade II) and restrictive (grade III) filling patterns. The characterization of diastolic dysfunction may be difficult in patients with HOCM due to significant variations in mitral inflow patterns due to individual differences in compliance and relaxation.[16] Even after excluding patients with HOCM, on multivariate analysis diastolic dysfunction was not associated with POAF in our study.
Our report shares similarities with the study by Melduni et al. in that no single echocardiographic parameter of diastolic function was predictive of POAF on multivariate analysis. However, the studies differ in that we were unable to show an association between the grade of diastolic function and POAF. We used a single set of national guidelines[16] for the grading of diastolic function [Figure 1], whereas Melduni et al. used this set of guidelines as well as other previously published reports of significant parameters echocardiographic parameters. These slightly dissimilar protocols for grading diastolic function may explain the differences in our findings. However, it is important to highlight the fact the grading of diastolic function is an inexact science, and a given patient's echocardiographic findings may not conform to published algorithms for diastolic grading. Inter-institutional variability and reviewer subjectivity in the grading of diastolic function may make it difficult to include diastolic grade into global predictive models of POAF.
Our results also agree with those of Melduni et al. with respect to increasing left atrial volume as a predictor of POAF. The significance of this finding is difficult to assess. Left atrial enlargement accompanies chronic mitral stenosis and regurgitation, and patients in our study presenting for mitral valve surgery were more likely to experience POAF (P = 0.037). When patients undergoing mitral valve surgery were excluded, increased left atrial volume was no longer associated with POAF on multivariate analysis. Left atrial enlargement is also found in patients with advanced degrees of diastolic dysfunction,[16] though in the current study, there was no difference in the presence of advanced diastolic dysfunction between the two groups. Unlike the reports of Melduni, we also included initial intraoperative pulmonary artery systolic pressure in our model, which was predictive of POAF. Our findings of increasing age, left atrial size, and elevated pulmonary artery pressures being associated with POAF are similar to other studies.[1,10,14,20] Thus, it may be that more objective clinical sequelae of diastolic dysfunction such as pulmonary hypertension and left atrial dilation are more predictive of POAF than grading of diastolic dysfunction.
Postoperative atrial fibrillation increases costs associated with cardiac surgery by several thousand dollars and hospital length of stay by 1–6 days as observed in the current study.[1,7,13,14,20,21] Preventative treatment is of interest to mitigate an increase in morbidity, cost, and hospital length of stay associated with POAF. Multiple different prophylactic strategies have been studied in an attempt to decrease the occurrence of POAF. In a systematic review of randomized controlled trials of adults undergoing cardiac surgery, the following prophylactic interventions were found to significantly reduce the rate of POAF: Amiodarone, β-blockers, magnesium, sotalol, atrial pacing, and posterior pericardiectomy.[22] This review found that hospital cost was decreased by $1,250 and length of hospital stay decreased by two-thirds of a day in patients receiving effective prophylactic interventions.
Although not significantly different in effectiveness from β-blockade, amiodarone may be preferred over β-blockers in patients with contraindications to β-blockers or those with a concern over myocardial depression following postoperative β-blocker administration.[23] Prophylactic amiodarone administration exposes patients to risk of side effects such as pulmonary toxicity, QT prolongation, symptomatic bradycardia, atrioventricular block, thyroid toxicity, and hepatotoxicity.[24,25] In addition, although one meta-analysis demonstrated effectiveness of perioperative intravenous amiodarone (1 g/day for 2 days after operation) at preventing POAF after isolated coronary artery bypass grafting, in cardiac surgeries involving valve repair/replacement only preoperative orally administered amiodarone was effective at preventing POAF.[5,25] This example of POAF prophylaxis, along with its concomitant risk of medication-induced complications, emphasizes the value of models to predict patients at high risk for POAF. It is important that prophylactic strategies be applied to patients who may benefit the most while avoiding exposing patients at low risk of POAF to the potential side effects of unnecessary antiarrhythmic therapy. Patients in the current study were enrolled in a protocol designed to assess the impact of intraoperative amiodarone or lidocaine on ventricular fibrillation. Patients who received intravenous bolus administration of amiodarone up to 450 mg versus placebo in the original randomized trial[15] showed no benefit in freedom from POAF (P = 0.41).
Occurrence of new-onset POAF in 40.8% of patients following cardiac surgery is consistent with previous reports including those from own institution.[1,2,3,4,5,6,7,8,9,10,11,12] The fact that 88% of patients experiencing POAF were treated with amiodarone suggest that these were clinically-significant episodes of POAF.
Limitations of this study include all those inherit to a retrospective review. However, the data were maintained in a prospective database as part of a randomized trial. Being a tertiary referral center involves the additional limitation that patients may seek care at outside institutions for events in the interim between preoperative testing (e.g. transthoracic echocardiogram) and surgery. Since transthoracic echocardiograms are not routinely repeated in the days immediately prior to surgery, it is possible that patients may have experienced events in this interim that may have changed their baseline diastolic function at the time of surgery. Although this study included patients with HOCM, a condition that renders diastolic function determination difficult, even after exclusion of these patients, diastolic dysfunction was not associated with POAF. This study is also limited in that various interventions known to prevent POAF such as postoperative beta blockade, atrial pacing, etc., were not examined. In addition, although amiodarone may be used to treat postoperative arrhythmias other than atrial fibrillation (e.g. ventricular arrhythmias or other supraventricular arrhythmias), this study was not designed to study the differences in incidences of these arrhythmias among patients receiving prophylactic intraoperative amiodarone compared to placebo.
CONCLUSIONS
In this relatively large patient series, diastolic dysfunction was not associated with POAF following a variety of cardiac surgeries. Diastolic dysfunction may not be a predictor of POAF as only one previous study has shown this association. Therefore, caution should be exercised before including diastolic dysfunction in predictive models of POAF. Our study is consistent with other reports showing that increasing age, left atrial size, and elevated pulmonary artery pressures are predictors of POAF.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Almassi GH, Schowalter T, Nicolosi AC, Aggarwal A, Moritz TE, Henderson WG, et al. Atrial fibrillation after cardiac surgery: A major morbid event? Ann Surg. 1997;226:501–11. doi: 10.1097/00000658-199710000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Echahidi N, Mohty D, Pibarot P, Després JP, O’Hara G, Champagne J, et al. Obesity and metabolic syndrome are independent risk factors for atrial fibrillation after coronary artery bypass graft surgery. Circulation. 2007;116:I213–9. doi: 10.1161/CIRCULATIONAHA.106.681304. [DOI] [PubMed] [Google Scholar]
- 3.Halonen J, Hakala T, Auvinen T, Karjalainen J, Turpeinen A, Uusaro A, et al. Intravenous administration of metoprolol is more effective than oral administration in the prevention of atrial fibrillation after cardiac surgery. Circulation. 2006;114:I1–4. doi: 10.1161/CIRCULATIONAHA.105.000851. [DOI] [PubMed] [Google Scholar]
- 4.Halonen J, Halonen P, Järvinen O, Taskinen P, Auvinen T, Tarkka M, et al. Corticosteroids for the prevention of atrial fibrillation after cardiac surgery: A randomized controlled trial. JAMA. 2007;297:1562–7. doi: 10.1001/jama.297.14.1562. [DOI] [PubMed] [Google Scholar]
- 5.Mahoney EM, Thompson TD, Veledar E, Williams J, Weintraub WS. Cost-effectiveness of targeting patients undergoing cardiac surgery for therapy with intravenous amiodarone to prevent atrial fibrillation. J Am Coll Cardiol. 2002;40:737–45. doi: 10.1016/s0735-1097(02)02003-x. [DOI] [PubMed] [Google Scholar]
- 6.Majahalme S, Kim MH, Bruckman D, Tarkka M, Eagle KA. Atrial fibrillation after coronary surgery: Comparison between different health care systems. Int J Cardiol. 2002;82:209–18. doi: 10.1016/s0167-5273(01)00622-2. [DOI] [PubMed] [Google Scholar]
- 7.Mathew JP, Parks R, Savino JS, Friedman AS, Koch C, Mangano DT, et al. Atrial fibrillation following coronary artery bypass graft surgery: Predictors, outcomes, and resource utilization. MultiCenter Study of Perioperative Ischemia Research Group. JAMA. 1996;276:300–6. [PubMed] [Google Scholar]
- 8.Mauermann WJ, Nuttall GA, Cook DJ, Hanson AC, Schroeder DR, Oliver WC. Hemofiltration during cardiopulmonary bypass does not decrease the incidence of atrial fibrillation after cardiac surgery. Anesth Analg. 2010;110:329–34. doi: 10.1213/ANE.0b013e3181c76bd3. [DOI] [PubMed] [Google Scholar]
- 9.Prasongsukarn K, Abel JG, Jamieson WR, Cheung A, Russell JA, Walley KR, et al. The effects of steroids on the occurrence of postoperative atrial fibrillation after coronary artery bypass grafting surgery: A prospective randomized trial. J Thorac Cardiovasc Surg. 2005;130:93–8. doi: 10.1016/j.jtcvs.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 10.Zaman AG, Archbold RA, Helft G, Paul EA, Curzen NP, Mills PG. Atrial fibrillation after coronary artery bypass surgery: A model for preoperative risk stratification. Circulation. 2000;101:1403–8. doi: 10.1161/01.cir.101.12.1403. [DOI] [PubMed] [Google Scholar]
- 11.Creswell LL, Schuessler RB, Rosenbloom M, Cox JL. Hazards of postoperative atrial arrhythmias. Ann Thorac Surg. 1993;56:539–49. doi: 10.1016/0003-4975(93)90894-n. [DOI] [PubMed] [Google Scholar]
- 12.Melduni RM, Suri RM, Seward JB, Bailey KR, Ammash NM, Oh JK, et al. Diastolic dysfunction in patients undergoing cardiac surgery: A pathophysiological mechanism underlying the initiation of new-onset post-operative atrial fibrillation. J Am Coll Cardiol. 2011;58:953–61. doi: 10.1016/j.jacc.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 13.Tamis JE, Steinberg JS. Atrial fibrillation independently prolongs hospital stay after coronary artery bypass surgery. Clin Cardiol. 2000;23:155–9. doi: 10.1002/clc.4960230305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aranki SF, Shaw DP, Adams DH, Rizzo RJ, Couper GS, VanderVliet M, et al. Predictors of atrial fibrillation after coronary artery surgery. Current trends and impact on hospital resources. Circulation. 1996;94:390–7. doi: 10.1161/01.cir.94.3.390. [DOI] [PubMed] [Google Scholar]
- 15.Mauermann WJ, Pulido JN, Barbara DW, Abel MD, Li Z, Meade LA, et al. Amiodarone versus lidocaine and placebo for the prevention of ventricular fibrillation after aortic crossclamping: A randomized, double-blind, placebo-controlled trial. J Thorac Cardiovasc Surg. 2012;144:1229–34. doi: 10.1016/j.jtcvs.2012.06.039. [DOI] [PubMed] [Google Scholar]
- 16.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–33. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 17.Davies MJ, Pomerance A. Pathology of atrial fibrillation in man. Br Heart J. 1972;34:520–5. doi: 10.1136/hrt.34.5.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayashi H, Wang C, Miyauchi Y, Omichi C, Pak HN, Zhou S, et al. Aging-related increase to inducible atrial fibrillation in the rat model. J Cardiovasc Electrophysiol. 2002;13:801–8. doi: 10.1046/j.1540-8167.2002.00801.x. [DOI] [PubMed] [Google Scholar]
- 19.Verheule S, Sato T, Everett T, 4th, Engle SK, Otten D, Rubart-von der Lohe M, et al. Increased vulnerability to atrial fibrillation in transgenic mice with selective atrial fibrosis caused by overexpression of TGF-beta1. Circ Res. 2004;94:1458–65. doi: 10.1161/01.RES.0000129579.59664.9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rostagno C, La Meir M, Gelsomino S, Ghilli L, Rossi A, Carone E, et al. Atrial fibrillation after cardiac surgery: Incidence, risk factors, and economic burden. J Cardiothorac Vasc Anesth. 2010;24:952–8. doi: 10.1053/j.jvca.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 21.Speir AM, Kasirajan V, Barnett SD, Fonner E., Jr Additive costs of postoperative complications for isolated coronary artery bypass grafting patients in Virginia. Ann Thorac Surg. 2009;88:40–5. doi: 10.1016/j.athoracsur.2009.03.076. [DOI] [PubMed] [Google Scholar]
- 22.Arsenault KA, Yusuf AM, Crystal E, Healey JS, Morillo CA, Nair GM, et al. Interventions for preventing post-operative atrial fibrillation in patients undergoing heart surgery. Cochrane Database Syst Rev. 2013;1:CD003611. doi: 10.1002/14651858.CD003611.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu J, Wang C, Gao D, Zhang C, Zhang Y, Lu Y, et al. Meta-analysis of amiodarone versus ß-blocker as a prophylactic therapy against atrial fibrillation following cardiac surgery. Intern Med J. 2012;42:1078–87. doi: 10.1111/j.1445-5994.2012.02844.x. [DOI] [PubMed] [Google Scholar]
- 24.Santangeli P, Di Biase L, Burkhardt JD, Bai R, Mohanty P, Pump A, et al. Examining the safety of amiodarone. Expert Opin Drug Saf. 2012;11:191–214. doi: 10.1517/14740338.2012.660915. [DOI] [PubMed] [Google Scholar]
- 25.Chatterjee S, Sardar P, Mukherjee D, Lichstein E, Aikat S. Timing and route of amiodarone for prevention of postoperative atrial fibrillation after cardiac surgery: A network regression meta-analysis. Pacing Clin Electrophysiol. 2013;36:1017–23. doi: 10.1111/pace.12140. [DOI] [PubMed] [Google Scholar]