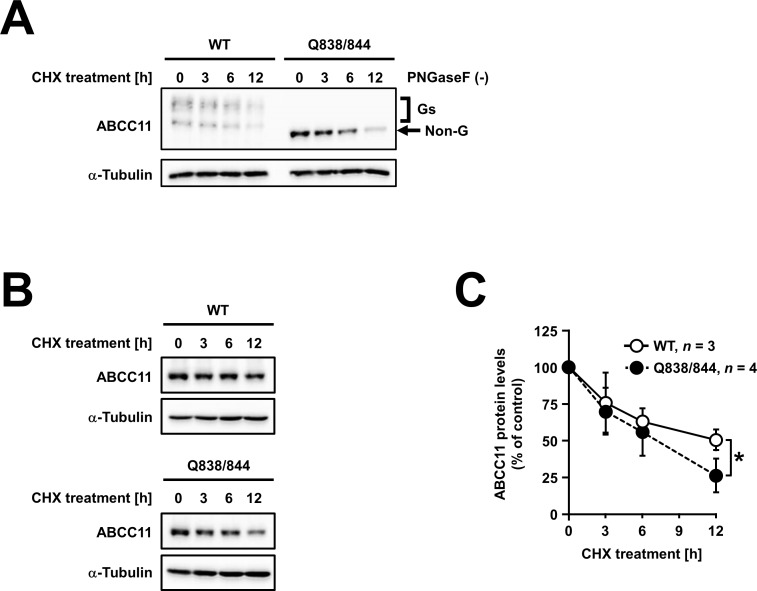
Fig 5. The effect of N-linked glycosylation on the protein stability of ABCC11.
(A and B) The effect of the inhibition of protein translation with cycloheximide (CHX) on the protein levels of ABCC11 wild-type (WT) and the Q838/844 mutant. Seventy-two hours after the transfection, MDCKII cells transiently expressing ABCC11 WT or Q838/844 were further incubated with 50 μM CHX for 0, 3, 6, and 12 h. At the indicated time, cells were collected and stored at -80°C until use. Simultaneously, all cell lysates were prepared and treated without (A) or with (B) PNGase F, and then subjected to immunoblotting. α-Tubulin: a loading control. The signal intensity ratio (ABCC11/α-tubulin) of the immunoreactive bands corresponding to the non-glycosylated form (Non-G) of ABCC11 protein was determined and normalized to the control (t = 0 h) level. Gs, glycosylated forms. (C) Densitometoric analysis of protein levels of the ABCC11 WT and the Q838/844 mutant. Values are expressed as % of control (values at 0 h). Data are expressed as mean ± S.D. n = 3 (WT), 4 (Q838/844 mutant). Statistical analyses for significant differences were performed according to Student’s t test (*, P < 0.05 in indicated time points).