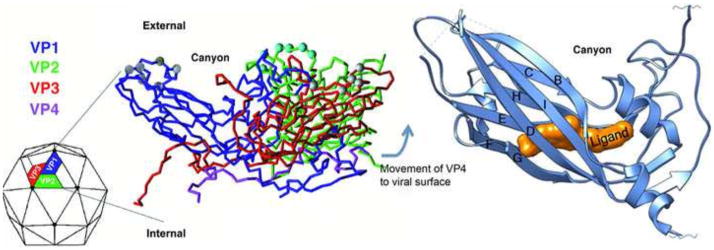
Figure 1. Structure of the picornavirus capsid and location of the hydrophobic pocket.
The left panel shows a color-coded C-alpha backbone model of the basic repeating unit of a picornavirus virion. The four capsid proteins, VP1, 2, 3, and 4 are color coded representing each of the proteins with VP4 being found on the interior of the particle apparently protected from immune surveillance. 60 copies of this repeating unit are organized as an icosahedron (shown as the particle on the left). At each vertex, five copies of VP1 come together and create a depression or “canyon” where many receptors have been shown to bind. The right panel shows a ribbon diagram of a picornavirus VP1 capsid protein showing the location of a small molecule inhibitor binding into the hydrophobic pocket normally occupied by pocket factor. The binding site is underneath the canyon floor and binding of inhibitor limits the flexibility and dynamics of the particle.