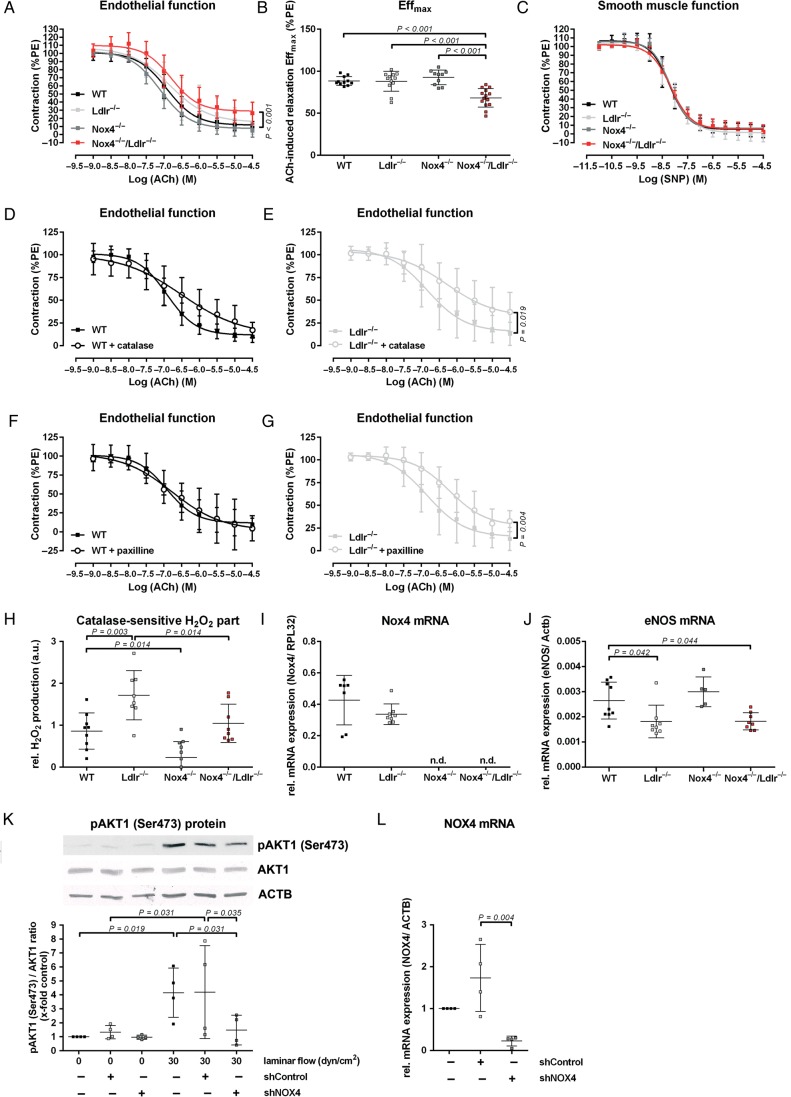
Figure 1.
Nox4−/−/Ldlr−/− mice develop endothelial dysfunction. (A) Concentration–response curve for acetylcholine in aortic segments of 10-week-old wild-type, Ldlr−/−, Nox4−/−, and Nox4−/−/Ldlr−/− mice (n ≥ 10) precontracted with phenylephrine. (B) Maximal effect of 30 µmol/L ACh on aortic segments of wild-type, Ldlr−/−, Nox4−/−, and Nox4−/−/Ldlr−/− mice (n ≥ 10). (C) Concentration–response curves for sodium nitroprusside in aortic segments of wild-type, Ldlr−/−, Nox4−/−, and Nox4−/−/Ldlr−/− mice (n ≥ 7). (D and E) Concentration–response curve for acetylcholine in aortic segments of 10-week-old wild-type and Ldlr−/− mice with catalase (n ≥ 9). (F and G) Concentration–response curve for acetylcholine in aortic segments of 10-week-old wild-type and Ldlr−/− mice with paxilline (n ≥ 9). (H) Amplex red assay for hydrogen peroxide generation of Aorta thoracalis segments of wild-type, Ldlr−/−, Nox4−/−, and Nox4−/−/Ldlr−/−. H2O2 formation was measured as the catalase-sensitive part of the signal (a.u.: arbitrary units) (n ≥ 8). (I and J) Real-time polymerase chain reaction of murine aorta from wild-type, Ldlr−/−, Nox4−/−, and Nox4−/−/Ldlr−/− mice (n ≥ 5). (K) Western blot analysis of pAKT1 (Ser473) in human umbilical vein endothelial cells transduced with shControl or shNOX4 for 48 h. Cells were either kept under static conditions or exposed to laminar shear stress (30 dyn/cm2) for 5 min (n=4. Statistics: one-way analysis of variance Duncan's method). (L) Real-time polymerase chain reaction of NOX4 in human umbilical vein endothelial cells. Cells were transduced for 72 h with shControl or shNOX4 (n = 4).