Fig. 1.
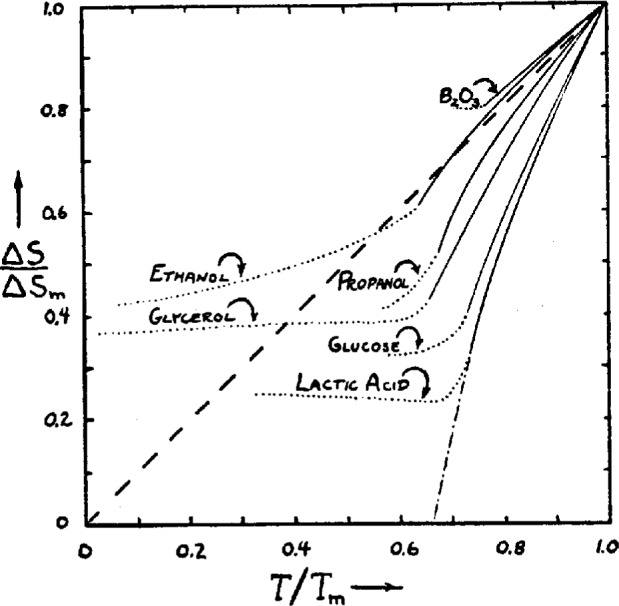
Kauzmann’s presentation of the entropy crisis which bears his name. The figure shows the rate at which the difference in entropy between liquid and crystal, normalized at the fusion point, disappears at T is lowered towards absolute zero. For B2O3, now known as a “strong liquid,” the liquid would always be of higher entropy than the crystal, even if the glass transition did not intervene at high T/Tm, to change the heat capacity. At the other extreme, lactic acid loses its excess entropy so rapidly on cooling that if Tg did not intervene to arrest the loss, liquid would arrive at the same entropy as the crystal at 2/3 of the melting point. (This is the temperature usually associated with the temperature of the glass transition itself (the 2/3 rule which this set of data only weakly support).) Lactic acid is an example of a “fragile” liquid. Other examples of these plots for fragile liquids are given in Ref. [2].
