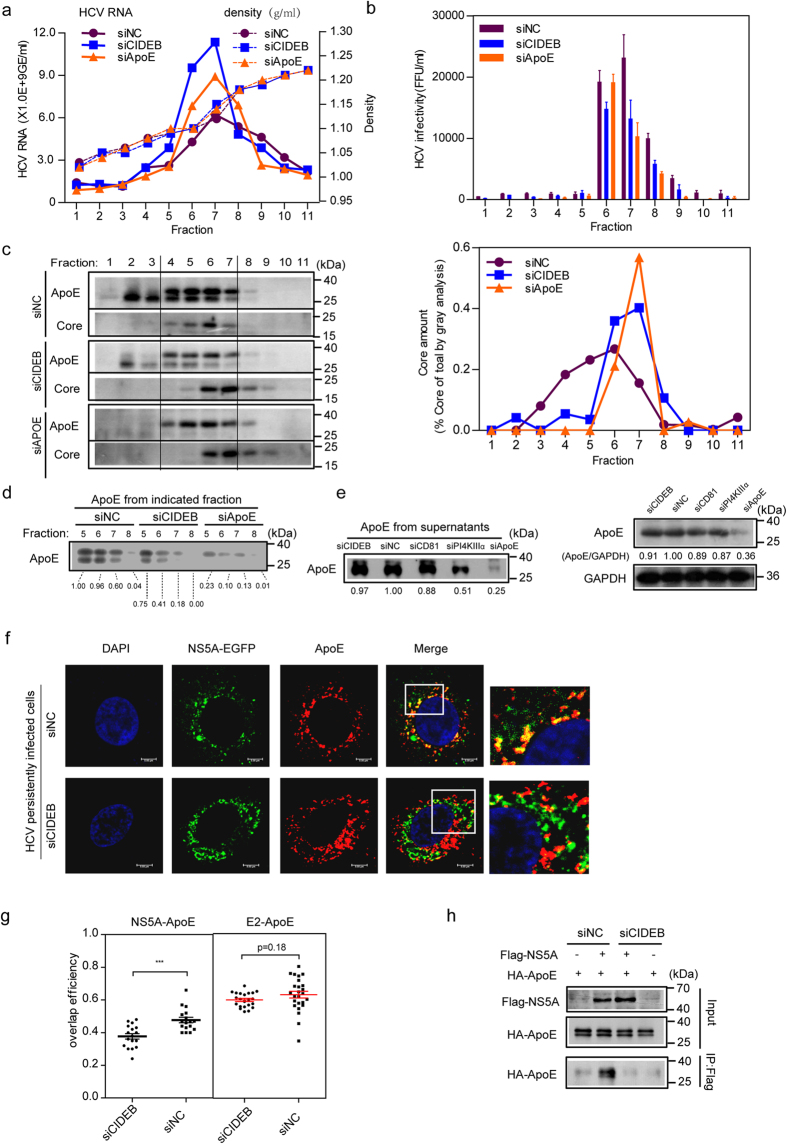
Figure 5. CIDEB silencing impairs the association of HCV particles with ApoE and disrupts the NS5A-ApoE interaction.
(a–c) Concentrated culture supernatants from HCV persistently infected Huh7.5.1 cells treated with siRNAs were subjected to 20–60% sucrose gradients and equilibrium ultracentrifugation. Eleven fractions were harvested from the top of the sucrose gradients and used to determine the density, HCV RNA levels, infectivity titer, and HCV core amounts. (a) The buoyant density of sucrose was plotted with the HCV RNA level as measured by qRT-PCR in each fraction. (b) The HCV infectivity of each fraction was evaluated. (c) Left: Western blot analysis of the ApoE and core protein levels in each fraction of the sucrose gradient. The right panel displays the relative core-to-total-core amounts as detected in all fractions. (d) Western blot analysis of ApoE from the indicated fractions of the sucrose gradient those were rich in HCV infectious particles. (e) Western blot analysis of total supernatants ApoE (left) and cell lysates ApoE (right). (f) The effect of CIDEB silencing on the co-localization of NS5A-EGFP with endogenous ApoE in HCV- Jc1EGFP persistently infected Huh7.5.1 cells. The magnification of the frame section of merged images is presented at the right of the merged images. (g) The effect of CIDEB silencing on NS5A-ApoE and E2-ApoE co-localization was evaluated by overlap efficiency. (h) The effect of CIDEB silencing on the NS5A-ApoE interaction. Huh7.5.1 cells were transduced with Flag-NS5A and HA-ApoE lentivirus for 48 h and then treated with siNC or siCIDEB for 72 h. Cell lysates were subjected to IP with an anti-Flag antibody and then immunoblotted with an anti-HA antibody.