Abstract
Background
KCNH1 encodes a voltage-gated potassium channel that is predominantly expressed in the central nervous system. Mutations in this gene were recently found to be responsible for Temple-Baraitser Syndrome (TMBTS) and Zimmermann-Laband syndrome (ZLS).
Methods
Here, we report a new case of TMBTS diagnosed in a Lebanese child. Whole genome sequencing was carried out on DNA samples of the proband and his parents to identify mutations associated with this disease. Sanger sequencing was performed to confirm the presence of detected variants.
Results
Whole genome sequencing revealed three missense mutations in TMBTS patient: c.1042G > A in KCNH1, c.2131 T > C in STK36, and c.726C > A in ZNF517. According to all predictors, mutation in KCNH1 is damaging de novo mutation that results in substitution of Glycine by Arginine, i.e., p.(Gly348Arg). This mutation was already reported in a patient with ZLS that could affect the connecting loop between helices S4-S5 of KCNH1 with a gain of function effect.
Conclusions
Our findings demonstrate that KCNH1 mutations cause TMBTS and expand the mutational spectrum of KCNH1 in TMBTS. In addition, all cases of TMBTS were reviewed and compared to ZLS. We suggest that the two syndromes are a continuum and that the variability in the phenotypes is the result of the involvement of genetic modifiers.
Keywords: Temple-Baraitser syndrome, Whole genome sequencing, KCNH1, Zimmermann-Laband syndrome
Background
Temple-Baraitser syndrome (TMBTS; MIM: 611816) and Zimmerman-Laband syndrome (ZLS; MIM: 135500) are rare developmental disorders with hypoplasia/aplasia of nails. These syndromes are considered to be distinct entities, with TMBTS defined as a disorder characterized by severe intellectual disability (ID), epilepsy, hypoplasia/aplasia of the nails of the thumb and great toe, a pseudo-myopathic appearance, and marked hypotonia in infancy [1–6], and ZLS charatacterized by ID, gingival fibromatosis, associated with absence or dysplasia of all nails, hypoplasia of the distal phalanges, scoliosis, hepato-splenomegaly, coarse face, and hirsutism [7].
KCNH1 encodes a voltage-gated potassium channel that is predominantly expressed in the central nervous system, and mutations in this gene have been linked to both syndromes [6, 7].
Here, we report on a Lebanese male patient with TMBTS having a mutation in KCNH1 that has previously been reported in a patient with ZLS. In addition, we have reviewed all published cases of TMBTS and highlight common features, as well as critical differences, between these two syndromes, and raise the issue of whether their classification into two entities is appropriate.
Methods
Clinical report
The male proband is the third child of healthy unrelated Lebanese parents. He was born at 36 weeks of gestation, after a complicated pregnancy characterized by the therapeutic administration, to the mother, of drugs against early contractions at 32 weeks of gestation. At birth, his weight was 2700 g (60th percentile), his length 48 cm (75th percentile) and his head circumference (OFC) 33 cm (60th percentile). Family history was unremarkable. Marked hypotonia, constipation, and aplasia of thumb and great toe nails were noted in the first two to three days of life.
The propositus was referred for genetic examination at the age of 9 months. His weight was 9750 g (60th percentile), length 71.5 cm (75th percentile), OFC 42.7 cm (10th percentile). He had a flat occiput, a frontal bossing, large ears, mild hypertelorism, epicanthal folds, a broad and depressed nasal bridge, a short columella, long philtrum, a broad mouth with downturned corners, a high arched palate, 2 upper and 2 lower incisors of normal shape, and full cheeks (Fig. 1). Widely spaced nipples and left chest depression were also noted. Both thumbs were held in an adducted posture and were terminally broad with aplasia of the nails bilaterally. Big toes were also broad, long, and with aplasia of nails. No hirsutism, no hypoplasia of the distal phalanges, no hypermobility, no camptodactyly, nor palmar creases were noted.
Fig. 1.
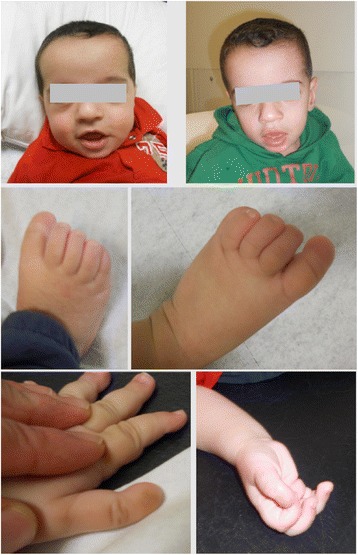
Photographs of the patient at the age of 9 and 15 months. Note the frontal bossing, mild hypertelorism, broad and depressed nasal bridge, broad mouth with downturned corners, full cheeks and the myopathic face. Both thumbs are held in an adducted posture are terminally broad with aplasia of the nails bilaterally. Big toes are also broad, long, with aplasia of nails
At 15 months old, his weight was 11 kg (75th percentile), length 79 cm (50th percentile), and OFC 45.7 cm (10th percentile). Delays in developmental milestones were striking, as he could not stand up alone or walk with help, and could not follow or respond to simple commands. He had a myopathic face with poor visual contact, a wide open mouth and mild gingival enlargement (Fig. 1). Skeletal survey revealed nearly absent distal phalanges of the thumbs and great toes, very small femoral and humeral epiphyses, and an osteosclerosis of the anterior arc of the right 10th rib (Fig. 2).
Fig. 2.
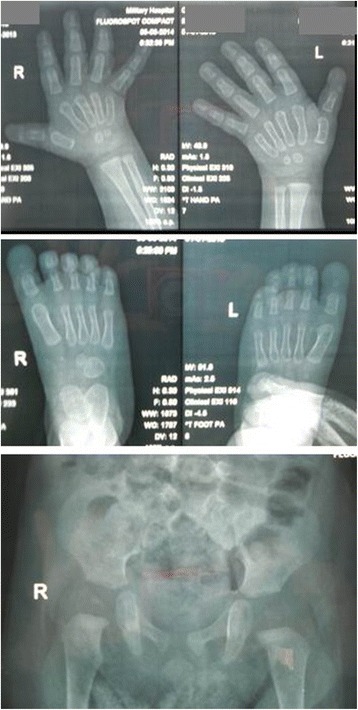
X-ray films of the patient. Note the absent distal phalanges of the thumbs and great toes, and the very small femoral epiphyses at the age of 15 months
Magnetic resonance imaging, abdominal and heart ultrasound, brain stem auditory evoked responses, and EEG were normal. Complete blood count, hemoglobin electrophoresis, serum electrolytes, blood glucose levels, urinalysis, thyroid, liver and renal function tests were all unremarkable. Array CGH analysis and Chromosomal Microarray Analysis did not reveal any abnormalities (data not shown).
DNA extraction and Whole Genome Sequencing (WGS)
Whole genome sequencing was carried-out on the patient and his parents using the HiSeq 2500 sequencer (Illumina, San Diego, CA, USA). Libraries were generated from 1 μg of genomic DNA [8] using the Illumina TruSeq DNA PCR-Free Sample Preparation Kit. Genomic DNA was sheared using the Covaris system (Woburn, MA, USA). Isolated DNA fragment ends were blunted, A-tailed and ligated with sequencing adaptors with index sequences. Excess adapters and enzymes were removed using AMPure beads (Beckman Coulter Genomics, Danvers, MA, USA). Indexed libraries were size selected to 350 bp range using bead-based capture and the concentration of amplifiable fragment was determined by qPCR relative to sequencing libraries with known concentration. Normalized libraries were clustered on a c-BOT machine and 125 bp paired-end sequencing was performed on the HiSeq2500 system.
WGS data analyses
Raw data was mapped to the human genome reference build 19 (http://www.broadinstitute.org/ftp/pub/seq/references/Homo_sapiens_assembly19.fasta) using BWA aligner [9] version 0.7.7-r441 and variant call was performed using GATK [10] version 3.3.2. The rare variant analysis was performed using the xbrowse tool (https://xbrowse.broadinstitute.org/). For the parents and the child, a ‘De novo Dominant’ inheritance model was selected, with severity of the variant effect set to ‘moderate to high impact’ (Nonsense, essential splice sites, missense frameshift and in frame), call quality as high (genotype quality > 20 and allele balance ratio > 25 %) and allele frequency < 1 % in 1000 genomes and The Exome Aggregation Consortium (ExAC) v0.3 datasets. Functional consequences of amino acid substitutions have been predicted using various tools [11–14].
Sanger sequencing
Genomic sequences of KCNH1, STK36, and ZNF517 were obtained from UCSC Genome Browser (December 2013). A flanking region around each sequence variant site was amplified by PCR with the following primer pairs: forward primer (5′-TCAACGCTTTTGAGAACGTG-3′) and reverse primer (5′-TGTCTTGGTGTCCTCGTCAA-3′) for KCNH1 (NM_002238); forward primer (5′-CATCCCTCATCTCTGGCCTG-3′) and reverse primer (5′-ACTTTTACCTTGCCCTGAATCA-3′) for STK36 (NM_001243313); and forward primer (5′-TTCAAGCAAAGCTCCATCCT-3′) and reverse primer (5′-GGTGTGGAACTTCTGGTGCT-3′) for ZNF517 (NM_213605). Primers for the PCR amplifications were designed using Primer3 Software. PCR reactions were performed using Taq DNA polymerase (Invitrogen Life Technologies, Carlsbad, CA, USA). PCR fragments were run on 1 % agarose gel. The fragments were purified using the Illustra_ GFX_ PCR DNA and Gel Band Purification Kit (GE Healthcare) and then sequenced using the Big Dye_ Terminator v 1.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA). Sequence reaction was purified on Sephadex G50 (Amersham Pharmacia Biotech, Foster City, CA), and then loaded into an ABI 3100 system after the addition of Hidi formamide. Electropherograms were analyzed using Sequence Analysis Software version 5.2 (Applied Biosystems) and then aligned with the reference sequences using ChromasPro version 1.22 (Technelysium, Queensland, Australia).
Results
Whole Genome Sequencing identified 3 missense mutations in TMBTS patient (Table 1). We validated and confirmed the de novo origin of these variants by Sanger sequencing.
Table 1.
Variants identified with the WGS analysis while running a de novo dominant model using xbrowse
| Gene | Position | Function | Software prediction |
|---|---|---|---|
| KCNH1 | chr1:211093321 | Missense | Polyphen: probably damaging |
| C > T | c.1042G > A | Sift: damaging | |
| p.(Gly348Arg) | Mutation taster: disease causing | ||
| Fathmm: damaging | |||
| STK36 | chr2:219558050 | Missense | Polyphen: possibly damaging |
| T > C | c.2131 T > C | Sift: damaging | |
| p.(Cys711Arg) | Mutation taster: disease causing | ||
| Fathmm: tolerated | |||
| ZNF517 | chr8:146033027 | Missense | Mutation taster:disease causing |
| C > A | c.726C > A | ||
| p.(Phe242Leu) |
The mutation in KCNH1 (c.1042G > A) has a damaging effect according to all different effect predictors tested. STK36 has a missense mutation (c.2131 T > C), which also has damaging effects according to half of the effect predictors tested. ZNF517 has a missense mutation (c.726C > A) predicted as disease causing by one of the effect predictors.
The KCNH1 mutation results in a substitution of Glycine by Arginine. Same mutation is found in both isoforms of this protein: p.(Gly348Arg) in short isoform (NM_002238.3) and p.(Gly348Arg) in long isoform (NM_172362) in the ion transport domain. The p.(Gly348Arg) mutation maps to the connecting loop between helices S4-S5 as reported by Kortum et al., and exerts a strong impact on function [18].
Discussion
We report on a male Lebanese patient in which a de novo missense heterozygous mutation c.1042G > A in the KCNH1 gene led to TMBTS.
KCNH1 is a member of voltage-gated potassium channel proteins. It is recognized as an important regulator of cell proliferation in bone-marrow derived mesenchymal stem cells, and is involved in fundamental cellular and developmental processes [15, 16].
Mutations in KCNH1 have been recently associated with TMBTS [6]. Moreover, de novo gain-of-function mutations in KCNH1 have also been reported in individuals with ZLS [7].
Generally, TMBTS and ZLS can be distinguished by their characteristic phenotypic features, which include absence or dyplasia of all nails and hypertrichosis in ZLS vs hypoplasia or aplasia of only the great toe and thumb’s nails in TMBTS (Table 2). With this in mind, we considered that our patient had TMBTS. These syndromes are currently considered to be two separate entities, but their common characteristics suggest that these two syndromes may be different presentations of the same disorder. In fact, many common characteristics of patients with TMBTS and ZLS have been noted, such as, seizures, hypertrichosis, hypotonia, aplasia of nails, etc., which sometimes occur in some but not all patients (Table 2). It is noteworthy to mention that many clinical databases do not even mention TMBTS as a differential diagnosis for ZLS because of the absence of hypertrichosis, even though not all reported patients with ZLS present this characteristic.
Table 2.
Review of all cases with the Temple-Baraitser Syndrome and a comparison to the Zimmermann-Laband syndrome characteristics
| Temple Baraitser Syndrome (TMBTS) | Zimmermann-Laband syndrome (ZLS) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Present Patient | Temple and Baraitser (1991) [1] | Gabbett et al. (2008) [2] or Simons et al. (2014) Patient A | Jacquinet et al. (2010) [3] Patient 1 or Simons et al. (2014) Patient D | Jacquinet et al. (2010) [3] Patient 2 or Simons et al. (2014) Patient E | Yesil et al. (2013) or Simons et al. (2014) Patient C | Shen (2015) [5] Patient 1 or Simons et al. (2014) Patient F | Shen (2015) [5] Patient 2 or Simons et al. (2014) Patient B | Total of affected patients with TMBTS with KCNH1 mutations | Castori et al.(2013) | Zimmermann-Laband syndrome Kortüm et al. (2015) [18] KCNH1 mutations |
Zimmermann-Laband syndrome Kortüm et al. (2015) [18] ATP6V1B2 mutations |
Bramswig et al. (2015) [19] Individual 1 |
Bramswig et al. (2015) [19] Individual 2 |
Bramswig et al. (2015) [19] Individual 3 |
Bramswig et al. (2015) [19] Individual 4 |
|
| Complicated Prgenancy | + | - | + | - | - | - | - | + | ND | ND | ND | ND | ND | ND | ND | |
| Milestone | ||||||||||||||||
| Birth weight | 2,700 g (60th percentile) | 3,370 ga | 3,980 g (90th centile) | 3,590 g (50th centile) | 2,980 g (40th centile) | 3,600 g (50th percentile) | 7 pounds 7 ounces | 3,544 g (50th centile) | 2,710 g | 2,850 g | 3,354 g | NA | ||||
| Height at birth | 48 cm (75th percentile) | ND | ND | 45 cm (10th centile) | 52 cm (50-75 percentile) | 45 cm | 50 cm | 52 cm | NA | |||||||
| Head circumference at birth | 33 cm (60th percentile) | 35.5 cma | ND | 34 cm (30th centile) | 33 cm (40th centile) | 34 cm | 35 cm | NA | NA | |||||||
| Clinical findings | ||||||||||||||||
| Age (years) | 09/12 | 35/12 | 44/12 | 610/12 | 11/12 | 37/12 | 09/12 | 56/12 | 141/12 | 44/12 | 39/12 | 13 | ||||
| Consanguinity | - | - | - | - | - | - | - | - | - | - | - | - | ||||
| Limbs | ||||||||||||||||
| Absence/hypoplasia of thumb nail | + | + | + | + | + | + | + | + and of all fingers | 8/8 | 52/52 Hypoplasia/aplasia of nails/phalanges | 5/6 Hypoplasia/aplasia of nails | 2/2 Hypoplasia/aplasia of nails | - | - | - | - |
| Absence/hypoplasia of hallux nail | + | + | + | + | + | + | + | + | 8/8 | 5/6 | 2/2 | + | + | + | + | |
| Broad thumbs terminally | + | - | + | + | + | + | - | + | 6/8 | ND | 0/4 | ND | - | - | + | - |
| Thumbs; long/proximaly set | + | ND | + | + | + | + | + | + | 7/7 | ND | 3/4 | ND | + | - | - | + |
| Adductus deformity of distal thumb | + | ND | + | + | + | + | + | + | 7/7 | N D | ND | ND | ND | ND | ND | ND |
| Pseudoepiphysis of the thumb | - | - | + | + | + | ND | ND | ND | 3/5 | ND | ND | ND | ND | ND | ND | ND |
| Pseudoepiphysis of the great toe | - | ND | - | + | - | ND | ND | Absence of the secondary ossification center and longer great toes | ND | ND | ND | ND | ND | ND | ND | |
| Pseudoepiphysis of the distal thumb phalanges | - | + | + | + | + | + | no but malpatterned | no but malpatterned | 5/8 | ND | ND | ND | ND | ND | ND | ND |
| Hypoplasia of distal phalanges (II-V) | - | + | + | - | + | + | + | + | 6/8 | 52/52 Hypoplasia/aplasia of nails/phalanges | 4/5 Hypoplasia/aplasia of terminal phalanges; 1 NA | 2/2 Hypoplasia/aplasia of terminal phalanges | ND | ND | ND | ND |
| Delay in epiphyseal maturation | + | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
| Neurologic | ||||||||||||||||
| Intellectual disability | + | + | + | + | N/A | + | + | + | 7/7 | 21/52 | 6/6 | 2/2 | + | + | + | + |
| Poor visual contact | + | + | ND | + | + | + | ND | ND | 5/5 | ND | ND | ND | ND | + | + | + |
| Autistic behavior | - | + | + | ND | ND | + | ND | ND | 3/4 | ND | ND | ND | ND | + | + | ND |
| Seizures | - | ND | + | + | + | + | One seizure | + | 6/7 | 7/52 | 6/6 | 0/2 (patients ages: 22 and 5 years) | + | - | + | + |
| Hypotonia/motor retardation | + | + | + | + | + | + | + | + | 8/8 | 6/52 | 6/6 | 2/2 | + | + | + | + |
| Occipitofrontal circumference (centile) | 10th | 10th | 25-50th | 25-50th | 25th | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
| Hearing loss | - | ND | ND | ND | ND | ND | ND | ND | 2/52 | 1/4 2 NA |
1/2 | ND | ND | ND | ND | |
| Abnormal MRI findings | - | Widespread cerebral atrophy | - | - | - | Mild frontotemporal atrophy | - | - | ND | 2/4 2 NA |
1/1 1 NA |
- | Hypoplastic corpus callosum, cystic lesion pineal gland | - | Cystic lesion pineal gland | |
| Dysmorphic features | ||||||||||||||||
| Thoracic abnormalities | + | ND | ND | ND | - | ND | ND | ND | 1 has Pectus carinatum and thoracic kyphosis. Others ND | 1 has pectus carinatum | ND | ND | ND | ND | ND | |
| Spine abnormalities | - | ND | ND | ND | ND | ND | ND | ND | 8/52 | 5/6 Scoliosis | 1 Scoliosis | ND | ND | ND | ND | |
| Coarse face | + | ND | ND | ND | ND | ND | ND | ND | at least 1. Others ND | 6/6 | 2/2 | ND | ND | + | ND | |
| Myopathic appearance | + | ND | + | + | + | + | + | + | 7/7 | ND | 4/5 | ND | + | + | + | + |
| Low anterior hairline | - | + | ND | ND | ND | ND | + | High anterior hairline | ND | 1/6 | ND | ND | ND | ND | ND | |
| Coarse thick hair | - | ND | + | Hypertrichosis | - | + | ND | ND | Facial hypertrichosis in 8/52, body hypertrichosis in 19/52 | Hypertrichosis 3/6 | Marked hypertrichosis 2/2 | + | + | + | + | |
| Flat forehead | Bulging | + | + | + | + | + | ND | ND | 5/6 | ND | ND | ND | Prominent | ND | Broad and prominent | ND |
| Mild hypertelorism | + | + | - | + | + | + | + | + | 7/8 | 6/52 | 4/5 | ND | + | + | + | + |
| Epicanthal folds | + | - | + | - | + | + | - | - | 4/8 | ND | 1/6 | ND | - | + | + | - |
| Broad depressed nasal bridge | + | + | + | + | + | + | + | + | 8/8 | ND | 3/4 depressed 5/5 broad |
ND | + | + | + | Only broad |
| Short columella | + | - | + | + | + | + | - | + | 6/8 | ND | 4/4 | ND | + | + | + | - |
| Long philtrum | + | + | + | + | + | + | + | + | 8/8 | ND | 2/6 1 short philtrum |
1/2 | ND | ND | ND | ND |
| Thick/full vermillion border of upper lip | - | ND | + | + | + | Upper and lower lip | Upper and lower lips | Tented vermilion of upper lip and everted thick vermilion of the lower lip | 5/7 | 27 thick lips/macrostomia | 5/6 | ND | + | + | + | + |
| Broad mouth with downturned corners | + | ND | + | + | + | + | + | + | 7/7 | ND | 4/4 | ND | + | + | + | + |
| Gingival enlargement | + | - | - | - | - | - | - | - | 1/8 | 52/52 | 5/6 | 2/2 | + | + | + | + |
| Narrow and high palate | + | ND | + | ND | ND | ND | + | ND | 11/52 | ND | ND | ND | ND | ND | ND | |
| Inverted nipples | Widely spaced | ND | ND | ND | ND | Widely spaced | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
| Systemic manifestations | ||||||||||||||||
| Gastrointestinal symptoms | Constipation | Early feeding difficulties with recurrent vomiting | - | Severe gastroesophageal reflux in the neonatal period | ND | Constipation | - | Constipation | ND | 3/6 have gastroesophageal reflux and/or constipation | ND | Constipation | Slight feeding problem | Constipation | Severe feeding problem | |
| Small genitalia/endocrine anomalies | - | ND | ND | ND | ND | + | ND | ND | 3/52 abnormal genitalia | ND | 1 has macroorchidism | ND | ND | ND | ND | |
| Cardiovascular system anomalies | - | ND | - | ND | ND | Atrial septal defect and mild pulmonary stenosis | ND | ND | 6/52 | ND | ND | - | ND | - | Open ductus bodalli | |
Abbreviations: +, present; −, absent; NA not analyzed, ND not documented, N/A not applicable, MRI magnetic resonance imaging
a no standard deviation noted
Interestingly, the same mutation (c.1042G > A) identified in our patient has never been reported with TMBTS, but was previously detected in patients with ZLS (patient 7 in Abo-Dalo et al. or subject 3 in Kortüm et al.) [17, 18]. This substitution leads to a gain of function effect and mutants carrying this mutation exhibit an accelerated channel activation and a slower deactivation [18]. Along with the previously identified p.(Ile494Val) misense variant in KCNH1, which was shared among individuals with TMBTS and ZLS, the genetic defect identified in our patient, i.e., p.(Gly348Arg) was found in patients bearing different phenotypes and thus supposedly different syndromes. This provides stronger evidence that both syndromes clearly overlap and could be a phenotypic continuum. In fact, the mutation c.1042G > A was found in a patient with ZLS who does not present with hypertrichosis, similar to the patient reported herein. However, the patient had in addition, aplasia of all nails of hands and feet, thoracic scoliosis, and infrequent seizures, which were not present in our patient who had a delay in epiphyseal maturation, (Table 3) a feature never reported before in both entities, and gingival enlargement. The latter is a characteristic not reported previously in TMBTS affected individuals, however it is a frequent feature in patients with mutations of KCNH1 (Bramswig et al.). Genetic modifiers, possibly involving the Na+ and Ca2+ channels, might block the KCNH1 channels and result in the gingival enlargement as it is observed in individuals treated with Na+ blocker phenytoin or Ca2+ channel blocker nifedipine [18].
Table 3.
Clinical comparison between the patient here described with TMBTS and the patient described by Kortüm et al. (subject 3)
| Patients having the p.(Gly348Arg) mutation | ||
|---|---|---|
| Present patient | Subject 3 in Kortüm et al. (2015) | |
| Gender | M | F |
| Complicated Pregnancy | + | ND |
| Milestone | ||
| Birth weight | 2.700 g (60th percentile) | 3,290 g (39 weeks) (54th percentile) |
| Height at birth | 48 cm (75th percentile) | 55 cm (99th percentile) |
| Head circumference at birth | 33 cm (60th percentile) | ND |
| Clinical findings | ||
| Age (years) | 09/12 | 19 |
| Consanguinity | - | ND |
| Limbs | ||
| Absence of nails | Nails of thumb and hallux | Nails of hands and feet |
| Broad, long thumbs terminally | + | ND |
| Adductus deformity of distal thumb | + | ND |
| Hypoplasia of terminal phalanges of hands and feet | Nearly absent | + |
| Delay in epiphyseal maturation | + | ND |
| Neurologic | ||
| Intellectual disability | + | Severe |
| Poor visual contact | + | ND |
| Seizures | - | Started in adolescence |
| Hypotonia/motor retardation | + | + |
| Hearing loss | - | - |
| Abnormal MRI findings | - | NA |
| Dysmorphic features | ||
| Thoracic abnormalities | + | Thoracic scoliosis |
| Coarse face | - | + |
| Myopathic appearance | + | ND |
| Hypertrichosis | - | - |
| Coarse thick hair | - | - |
| Flat forehead | Bulging | ND |
| Mild hypertelorism | + | ND |
| Epicanthal folds | + | ND |
| Broad depressed nasal bridge | + | ND |
| Short columella | + | ND |
| Long philtrum | + | ND |
| Thick vermillion border of upper lip | - | ND |
| Broad mouth with downturned corners | + | ND |
| Gingival enlargement | + | Noticed in childhood prior anticonvulsant treatment |
| Central incisors | + | + |
| Narrow and high palate | + | ND |
| Inverted nipples | Widely spaced | ND |
| Systemic manifestations | ||
| Gastrointestinal symptoms | Constipation | ND |
| Small genitalia/endocrine anomalies | - | Solitary renal cyst |
| Cardiovascular system anomalies | - | ND |
Abbreviations: +, present; −, absent; ND not documented
On the other hand, the patient reported by Kortum et al., developed seizures in adolescence, therefore one could speculate a late occurence of epilepsy in the patient described here with the same mutation. Yet, Bramswig et al. described 3 individuals presenting with an identical KCNH1 variant but with different clinical features with regard to epilepsy [19]. Consequently, the presence of a pathogenic KCNH1 variant alone could not allow for a prediction of occurence of epileptic seizure.
Other genetic modifiers could be responsible for the observed differences in clinical phenotype. We looked deeper at the results of the WGS and noticed mutations in two other genes STK36 and ZNF517, which were classified in some databases as possibly damaging. However, their significance remains to be elucidated. Recently, de novo mutations in STK36 have been identified in patients with epileptic encephalopathies [20]. Although our patient who has missense mutation in STK36 does not present with epilepsy at present, he might develop it in adolescence as in patient 3 in Kortum et al. Thus, concordant to previous reports, our data supports the evidence that the mutated KCNH1 is a major cause of TMBTS and ZLS, while other genes can act as disease modifying roles. Understanding the molecular mechanisms by which these genes exert disease modifying roles might help in the better understanding of the pathogenesis of these syndromes.
Finally, both ZLS and TMBTS patients with KCNH1 mutations show similar phenotypes. Nevertheless, two other ZLS patients were also described with mutations in the ATP6V1B2 gene that encodes a component of the vacuolar ATPase (V-ATPase). These mutations present a more pronounced phenotype characterized mostly by hypertrichosis and a coarser facial phenotype (Table 2). But due to the limited number of individuals described, a conclusion about whether probands with mutations involving ATP6V1B2 lead to a more severe syndrome might not be accurate. On the other hand, Kortüm et al. screened a cohort of 24 ZLS patients, of which only 8 had mutations in KCNH1 and ATP6V1B2 suggesting further the genetic heterogeneity in the ZLS disorder [18].
Conclusions
In summary, this study shows that the same KCNH1 mutation can lead to both ZLS and TMBTS. The phenotypic variability could be the result of a modifier gene or genes, and identification of such genes would be of great importance. A careful analysis of genetic polymorphisms in various loci should be taken into consideration for clinical diagnosis. Further investigations are needed to confirm if ATP6V1B2 mutations lead to a more severe phenotype.
Abbreviations
ID, intellectual disability; TMBTS, Temple-Baraitser syndrome; WGS, whole genome sequencing; ZLS, Zimmermann-Laband syndrome.
Acknowledgements
We thank the patient and his parents for their cooperation.
Funding
This study was supported by Weill Cornell Medicine-Qatar and Sidra Medical and Research Center, Qatar.
Availability of data and materials
Data from this study are freely available and can be obtained by contacting the corresponding author.
Authors’ contributions
AM carried out the clinical genetic diagnosis of the patient and collected blood samples. AM, FM, DM and LC made substantial contribution to conception, design, and analysis of data. AM, NC, LC, KS, and AC drafted the manuscript, its revisions for important intellectual content and interpretation of data. RTh, EW, ST, WL and KS carried out sample processing and DNA isolation. MLe, RA, RT, PJ and EW performed bioinformatics data analysis and validation. All authors have read and approved the final version of the manuscript and its submission for publication.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Written informed consent was obtained from legally authorized representatives of the patient (parental consent) to participate in this study and its publication and any accompanying images. A copy of the written consent is available for review by the Editor of this journal.
Ethical approval and consent to participate
This study has been approved by the Saint Joseph University of Beirut’s Committee on Clinical Investigation and conformed to the tenets of the Declaration of Helsinki. Written informed consent was obtained from legally authorized representatives of the patient (parental consent) to participate in this study and its publication and any accompanying images. A copy of the written consent is available for review by the Editor of this journal.
Contributor Information
André Mégarbané, FAX: +961 1 328032, Email: andre.megarbane@yahoo.fr.
Lotfi Chouchane, FAX: +974 44928330, Email: loc2008@qatar-med.cornell.edu.
References
- 1.Temple IK, Baraitser M. Severe mental retardation and absent nails of hallux and pollex. Am J Med Genet. 1991;41:173–5. doi: 10.1002/ajmg.1320410207. [DOI] [PubMed] [Google Scholar]
- 2.Gabbett MT, Clark RC, McGaughran JM. A second case of severe mental retardation and absent nails of hallux and pollex (Temple-Baraitser syndrome) Am J Med Genet A. 2008;146A:450–2. doi: 10.1002/ajmg.a.32129. [DOI] [PubMed] [Google Scholar]
- 3.Jacquinet A, Gérard M, Gabbett MT, Rausin L, Misson J-P, Menten B, et al. Temple-Baraitser syndrome: a rare and possibly unrecognized condition. Am J Med Genet A. 2010;152A:2322–6. [DOI] [PubMed]
- 4.Yesil G, Guler S, Yuksel A, Alanay Y. Report of a patient with Temple-Baraitser syndrome. Am J Med Genet A. 2014;164A:848–51. doi: 10.1002/ajmg.a.36344. [DOI] [PubMed] [Google Scholar]
- 5.Shen JJ. Two cases of Temple-Baraitser syndrome: natural history and further delineation of the clinical and radiologic phenotypes. Clin Dysmorphol. 2015;24:55–60. doi: 10.1097/MCD.0000000000000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simons C, Rash LD, Crawford J, Ma L, Cristofori-Armstrong B, Miller D, et al. Mutations in the voltage-gated potassium channel gene KCNH1 cause Temple-Baraitser syndrome and epilepsy. Nat Genet. 2015;47:73–7. [DOI] [PubMed]
- 7.Castori M, Valiante M, Pascolini G, Leuzzi V, Pizzuti A, Grammatico P. Clinical and genetic study of two patients with Zimmermann-Laband syndrome and literature review. Eur J Med Genet. 2013;56:570–6. doi: 10.1016/j.ejmg.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler Transform. Bioinformatics. 2009;25:1754–60. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DePristo M, Banks E, Poplin R, Garimella K, Maguire J, Hartl C, et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet. 2011;43:491–8. [DOI] [PMC free article] [PubMed]
- 11.Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, et al. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7:248–9. [DOI] [PMC free article] [PubMed]
- 12.Kumar P, Henikoff S, Ng PC. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc. 2009;4:1073–81. doi: 10.1038/nprot.2009.86. [DOI] [PubMed] [Google Scholar]
- 13.Schwarz JM, Rodelsperger C, Schuelke M, Seelow D. Mutation Taster evaluates disease-causing potential of sequence alterations. Nat Methods. 2010;7:575–6. doi: 10.1038/nmeth0810-575. [DOI] [PubMed] [Google Scholar]
- 14.Shihab HA, Gough J, Cooper DN, Day IN, Gaunt TR. Predicting the functional consequences of cancer-associated amino acid substitutions. Bioinformatics. 2013;29:1504–10. doi: 10.1093/bioinformatics/btt182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ouadid-Ahidouch H, Le Bourhis X, Roudbaraki M, Toillon RA, Delcourt P, Prevarskaya N. Changes in the K+ current-density of MCF-7 cells during progression through the cell cycle: possible involvement of a h-ether.a-gogo K+ channel. Receptors Channels. 2001;7:345–56. [PubMed] [Google Scholar]
- 16.Hemmerlein B, Weseloh RM, Mello de Queiroz F, Knötgen H, Sánchez A, Rubio ME, et al. Overexpression of Eag1 potassium channels in clinical tumours. Mol Cancer. 2006;5:41. [DOI] [PMC free article] [PubMed]
- 17.Abo-Dalo B, Roes M, Canún S, Delatycki M, Gillessen-Kaesbach G, Hrytsiuk I, et al. No mutation in genes of the WNT signaling pathway in patients with Zimmermann-Laband syndrome. Clin Dysmorphol. 2008;17:181–5. [DOI] [PubMed]
- 18.Kortüm F, Caputo V, Bauer CK, Stella L, Ciolfi A, Alawi M, et al. Mutations in KCNH1 and ATP6V1B2 cause Zimmermann-Laband syndrome. Nat Genet. 2015;47:661–7. [DOI] [PubMed]
- 19.Bramswig NC, Ockeloen CW, Czeschik JC, van Essen AJ, Pfundt R, Smeitink J, et al. ‘Splitting versus lumping’: Temple-Baraitser and Zimmermann-Laband Syndromes. Hum Genet. 2015;134:1089-97. [DOI] [PubMed]
- 20.Epi4K Consortium; Epilepsy Phenome/Genome Project. Allen AS, Berkovic SF, Cossette P, et al. De novo mutations in epileptic encephalopathies. Nature. 2013;501:217–21. doi: 10.1038/nature12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data from this study are freely available and can be obtained by contacting the corresponding author.


