Fig. 1.
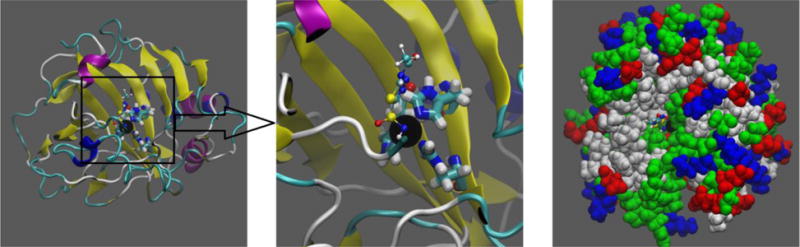
Structure of the hCAII-Zn2+-AZM complex. Shown in the left panel and the center panel (zoom into the active site) are the protein in ribbons colored by its secondary structures (beta sheets, yellow; helices, blue and purple; and coils, cyan and white). Also shown in these two panels are the zinc finger residues His 94, 96, and 119 (in licorices, colored by atom names) that tetrahedrally coordinate Zn2+ (large black sphere) along with AZM (in balls and sticks, colored by atom names). The color-by-atom names scheme: H, white; O, red; C, cyan; N, blue; S, yellow. Shown in the right panel are the protein and Zn2+ (in large spheres, colored by residue types: hydrophilic, green; hydrophobic, white; positively charged, blue; negatively charged, red) and AZM (in balls and sticks, colored as in the left panels) to illustrate the binding pocket. All molecular graphics in the paper were rendered with VMD.[11] The initial structure of the complex was from the RCSB Protein Data Bank (PDB code: 3HS4 [12]).
