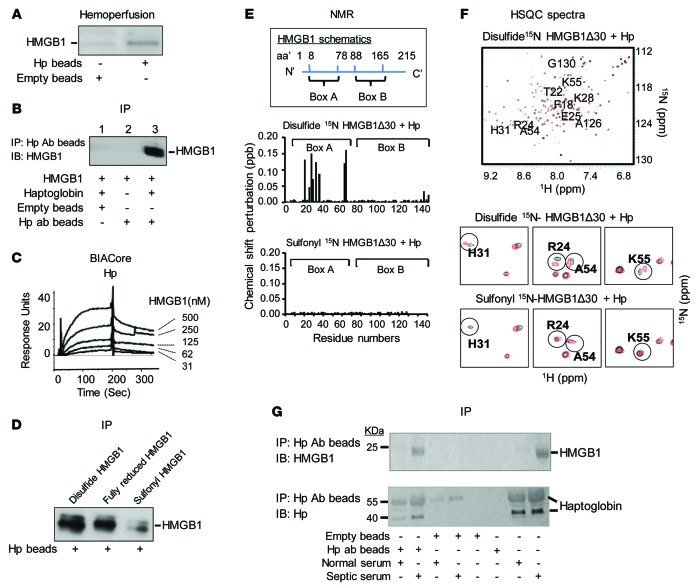
Figure 1. Haptoglobin (Hp) binds HMGB1.
(A) Rats were subjected to CLP to induce sepsis and subsequently connected to an extracorporeal pump to filter blood through human haptoglobin–conjugated (Hp) or empty beads for 1 hour. Proteins bound to Hp or empty beads were eluted and subjected to Western blot analysis using anti-HMGB1 antibodies. Data are representative from 3 repeats. (B) Human Hp and HMGB1 was mixed and applied to beads coupled with anti–human Hp antibody or control (empty beads). Proteins bound to the beads were analyzed by Western blot probed with anti-HMGB1 antibodies. Data are representative from 5 experiments. (C) Human Hp was immobilized on the sensor chip, and binding to HMGB1 was assessed. The apparent Kd is ~64 nM. Data are presented as response units over time (Sec) and representative of 3 experiments. (D) Human Hp–coupled beads were incubated with various HMGB1 isoforms. Elutes were analyzed by Western blot using anti-HMGB1 mAbs. Data are representative from 6 repeats. (E) Upper: Schematics of human HMGB1 structure. Middle/lower: nuclear magnetic resonance (NMR) analysis of disulfide (middle) or sulfonyl (lower) Δ30-HMGB1 in complex with human Hp. Chemical shift perturbation of disulfide or sulfonyl Δ30-HMGB1 in complex with Hp is plotted as a function of residue number of HMGB1. Perturbations in chemical shift above 0.1 are considered a significant interaction (46). The majority of interacting amino acids are located within HMGB1 Box A. Data are representative from 3 repeats. (F) Upper: 15N-1H-heteronuclear single quantum coherence (HSQC) spectra for disulfide Δ30-HMGB1 in the presence or absence of Hp. HMGB1 residues (10 amino acids) showing significant chemical shift perturbations due to Hp binding are labeled. Middle: Representative 15N-HSQC spectra of disulfide Δ30-HMGB1 free (black) and in complex with haptoglobin (red); residues showing chemical shift changes upon complex formation are circled. Lower: 15N-HSQC spectra of sulfonyl Δ30-HMGB1 free (black) and in complex with Hp (red). Residues showing chemical shift changes seen with disulfide Δ30-HMGB1 upon complex formation with Hp are circled for comparison. Nonoverlapping black and red resonance spectra indicate a significant chemical shift and, hence, an interaction between proteins via that amino acid. (G) Serum samples (10 μl) from healthy individuals or sepsis patients were incubated with beads conjugated to anti–human Hp mAbs or empty (20 μl drained beads). Eluates were probed with anti-HMGB1 (upper) and anti–human Hp (lower) antibodies using Western blot analysis. Data are representative from 5 samples each.