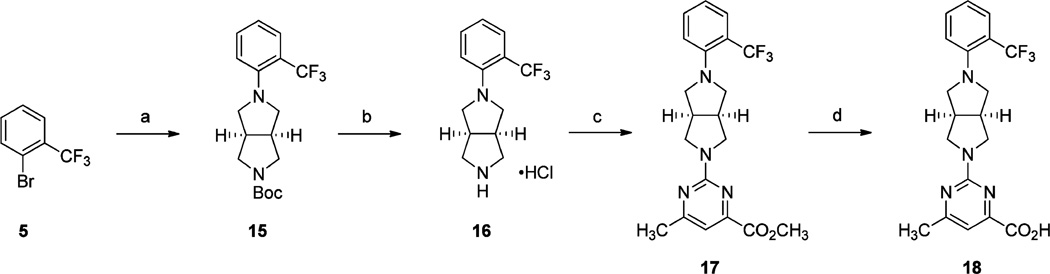
Scheme 3a.
aReagents and conditions: (a) (3aR,6aS)-tert-butyl hexahydropyrrolo[3,4-c]pyrrole-2(1H)-carboxylate, Pd(OAc)2, BINAP, NaOt-Bu, toluene, 110 °C, 16 h; (b) 2.0 M HCl solution in Et2O, CH2Cl2, rt, 3 h; (c) methyl 2-chloro-6-methylpyrimidine-4-carboxylate, i-Pr2NEt, DMF, 60 °C, 16 h; (d) (i) LiOH·H2O, H2O, THF, rt, 16 h; (ii) 2 N aq HCl.