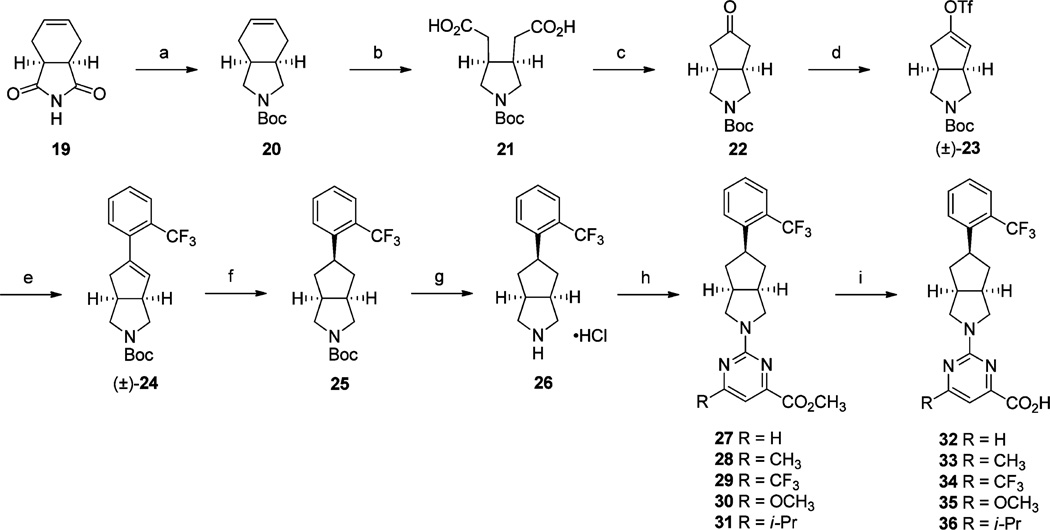
Scheme 4a.
aReagents and conditions: (a) (i) LiAlH4 (1.0 M solution in THF), THF, 70 °C, 16 h; (ii) Boc2O, CH2Cl2, rt, 16 h; (b) (i) NaIO4, RuO2·H2O, CH3CN, CCl4, H2O, rt, 24 h; (c) Ac2O, NaOAc, 120 °C, 3 h; (d) (i) LiHMDS (1.0 M solution in THF), THF, −78 °C, 30 min; (ii), PhN(SO2CF3)2, THF, −78 °C to rt, 3 h; (e) (2-(trifluoromethyl)phenyl)boronic acid, Pd(PPh3)4, 2 M Na2CO3, DME, 80 °C, 6 h; (f) H2 (40 psi), 10% Pd/C, CH3OH, rt, 16 h; (g) 2.0 M HCl in Et2O, CH2Cl2, 0 °C to rt, 24 h; (h) 2-chloro-6-substitutedpyrimidine-4-carboxylic acid methyl ester, Et3N, DMF, 60 °C, 16 h; (i) (i) 2 N aq NaOH, THF, CH3OH, rt, 16 h; (ii) 2 N HCl.