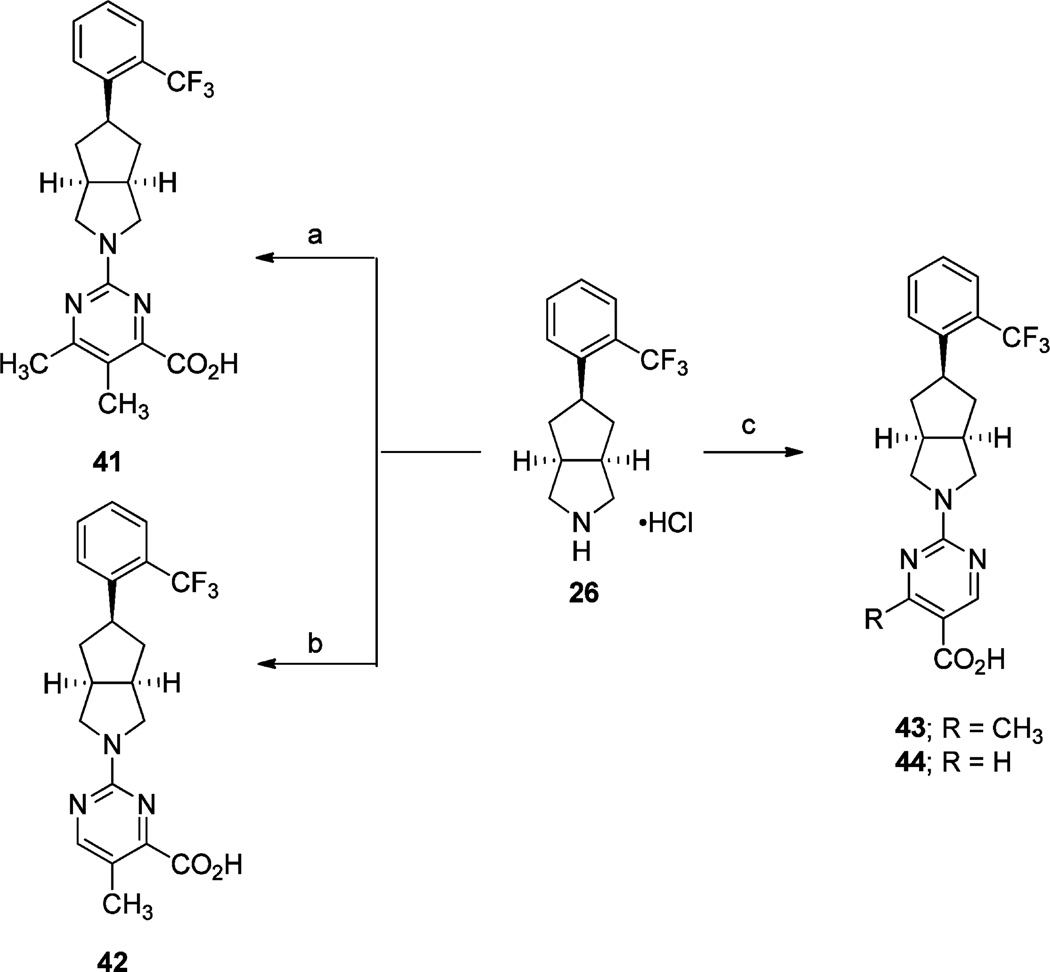
Scheme 6a.
aReagents and conditions: (a) (i) 2,4-dichloro-5,6-dimethylpyrimidine, Pd(OAc)2, Cs2CO3, JohnPhos, toluene, reflux, 16 h; (ii) Mo(CO)6, Pd(OAc)2, BINAP, CH3OH, CH3CN, 80 °C, 16 h; (iii) LiOH·H2O, THF, CH3OH, H2O, rt, 16 h; (b) (i) methyl 2-chloro-5-methylpyrimidine-4-carboxylate, Et3N, DMF, 60 °C, 16 h; (ii) 2 N aq NaOH, THF, CH3OH, rt, 16 h; (c) (i) methyl 2-chloro-4-methylpyrimidine-5-carboxylate or methyl 2-chloropyrimidine-5-carboxylate, i-Pr2NEt, 70 °C (microwave irradiation), 1.5 h or Et3N, DMF, 70 °C, 16 h; (ii) LiOH·H2O, THF, H2O, rt, 16 h or 2 N aq NaOH, THF, CH3OH, rt, 16 h; (iii) 2 N aq HCl.