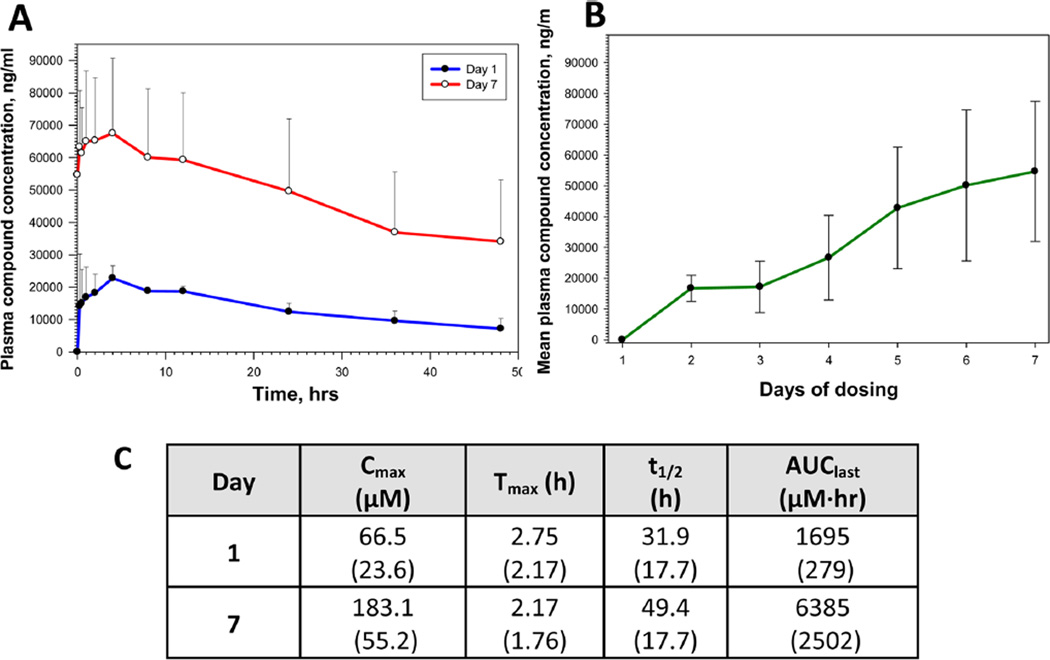
Figure 6.
(A) Plasma exposure levels of analogue 33 in Sprague–Dawley rats following a single (Day 1) and 7-day (Day 7) QD oral dose administration at 18.2 µmol/kg (5 mg/kg). Data represented as the mean (SD). Each dosing group consisted of three drug naïve adult male Sprague–Dawley rats; the test article vehicle was 2% Tween 80 in 0.9% saline. The difference in plasma compound concentration between Day 1 and Day 7 samples was statistically significant at all time points studied (P < 0.05; two-tailed unpaired Student’s t test). (B) Plasma concentration of analogue 33 at predose time points during the 7-day QD oral dose administration in three rats from Group 3. Data represented as the mean (SD). The predose compound concentration was statistically significantly increased at Day 7 in comparison to the Day 2 predose level (P < 0.05; two-tailed unpaired Student’s t test). (C) Pharmacokinetic parameters for 33 in Sprague–Dawley rats after a single dose (Day 1) and seven QD oral doses (Day 7) at 18.2 µmol/kg. Data are represented as the mean with standard deviation shown in parentheses.