Abstract
Recent epidemiological studies suggest a strong association between exposure to environmental contaminants, including organochlorine (OC) insecticides or their metabolites, and development of pathologies, such as atherosclerosis, in which oxidative stress plays a significant etiological role. Biomarkers of systemic oxidative stress have the potential to link production of reactive oxygen species (ROS), which are formed as a result of exposure to xenobiotic toxicants, and underlying pathophysiological states. Measurement of F2-isoprostane concentrations in body fluids is the most accurate and sensitive method currently available for assessing in vivo steady-state oxidative stress levels. In the current study, urinary concentrations of F2-isoprostanes and serum levels of persistent OC compounds p,p’-dichlorodiphenyldichloroethene (DDE), trans-nonachlor (a component of the technical chlordane mixture) and oxychlordane (a chlordane metabolite) were quantified in a cross-sectional study sample and the association of these factors with a clinical diagnosis of atherosclerosis determined. Urinary isoprostane levels were not associated with atherosclerosis or serum concentrations of OC compounds in this study sample. However, occurrence of atherosclerosis was found to be associated with serum trans-nonachlor levels. DDE and oxychlordane were not associated with atherosclerosis. This finding supports current evidence that exposure to environmental factors is a risk factor for atherosclerosis in addition to other known risk factors.
Introduction
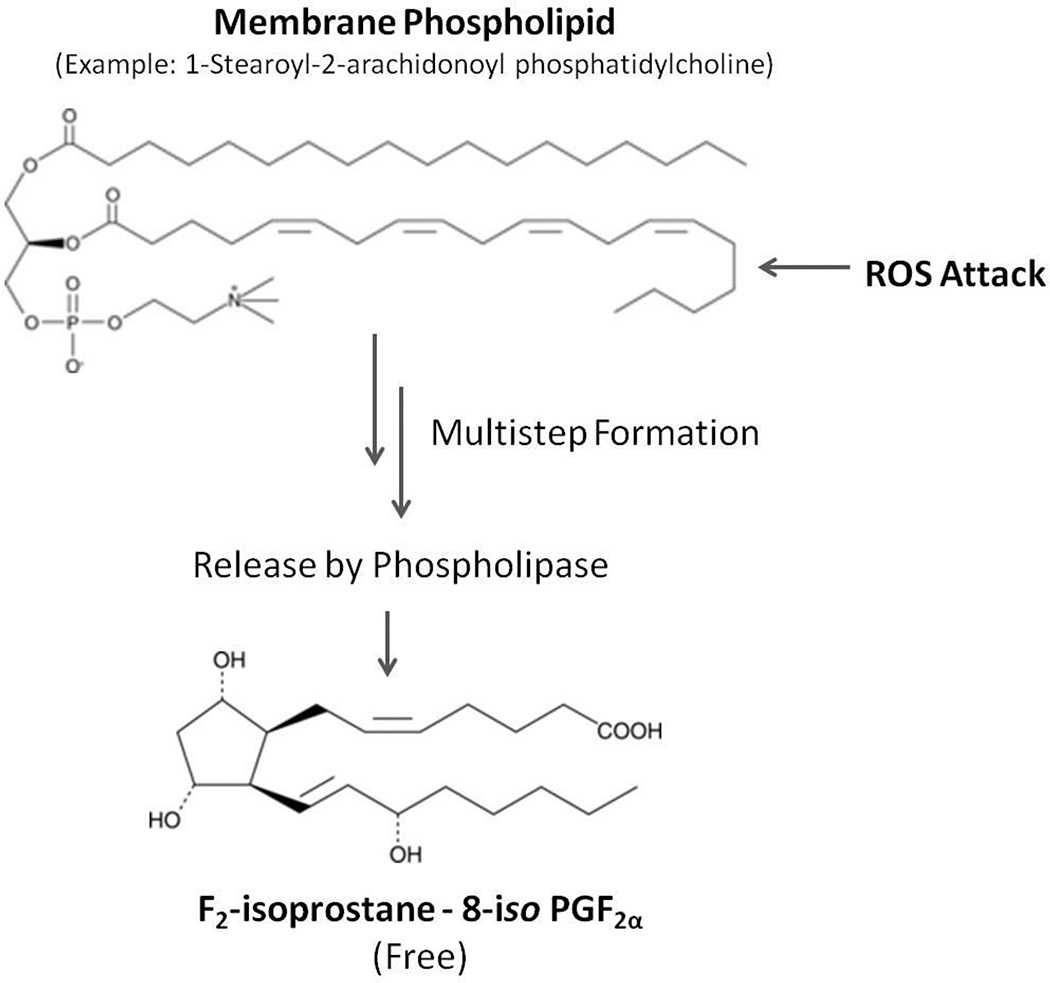
Biomarkers of systemic oxidative stress have the potential to link production of reactive oxygen species (ROS) that are derived from exposure to xenobiotic toxicants and underlying pathophysiological states. The measurement of F2-isoprostane concentrations in body fluids is the most accurate and sensitive method currently available for assessing in vivo steady-state systemic oxidative stress levels (Roberts and Morrow, 2000; Kadiiska et al., 2005; Milne et al., 2007). Isoprostanes are stable prostaglandin-like molecules formed in situ during free radical peroxidation of arachidonic acid, a component of low-density lipoprotein (LDL) and plasma membranes, which are subsequently released by phospholipases (Figure 1) (Morrow et al., 1992). Non-esterified F2-isoprostanes may be excreted unchanged in urine and due to their chemical stability, their levels can be assayed (Milne et al., 2011). Levels of isoprostanes in body fluids are increased following exposure to diverse chemical agents capable of producing oxidative damage to tissue, including tobacco smoke, carbon tetrachloride, nitrogen dioxide, and halothane (Morrow et al., 1992; 1995; Kharasch et al., 2000; de Broucker et al., 2015), whereas concentrations decrease in response to elevated blood antioxidant levels (Pratico et al., 1998; Davi et al., 2004). Risk factors for cardiovascular disease (CVD), such as smoking, obesity and type 2 diabetes (T2D), are known to elevate F2-isoprostane levels in body fluids (Milne et al., 2007; Morrow, 2005). Elevated levels of isoprostanes within atherosclerotic plaques suggest that isoprostanes may be causally involved in atherosclerosis disease development (Gniwotta et al., 1997). The isoprostane 8-iso-PGF2α binds to thromboxane receptors on endothelial cells inducing enhanced monocyte adhesion to vascular endothelium, resulting in enhanced monocyte infiltration into the arterial intima (Leitinger et al., 2001) thereby contributing to plaque formation and atherosclerosis.
Figure 1.
Schematic representation of F2-isoprostane formation. F2-isoprostanes are formed in situ in phospholipid membranes via peroxidation of phosphatidylcholine arachidonic acid through a multistep process. Isoprostanes may subsequently be released by phospholipase hydrolytic activity. ROS, reactive oxygen species.
Recent epidemiological studies suggested a strong association between human exposure to environmental contaminants, such as organochlorine (OC) compounds and development of pathologies, such as atherosclerosis and T2D, in which oxidative stress plays a significant etiological role (Lee et al., 2006a; 2006b; Longnecker and Daniels, 2001; Wang et al., 2010; Min et al., 2011; Ha et al., 2007). Although the use of bioaccumulative OC compounds like DDT and chlordane has been banned in the United States for decades, these legacy OC compounds and/or their metabolites and degradation products are still detected in serum samples in human populations within the United States and abroad, as well as in the US food supply (CDC, 2009; Eden et al., 2014).
In vivo animal studies demonstrated the ability of persistent OC compounds to contribute to T2D and CVD by perturbing normal glucose and lipid metabolism (Ruzzin et al., 2010). In another in vivo feeding study, male Sprague-Dawley rats dosed with trans-nonachlor displayed significant elevation in liver oxidative stress biomarkers following 90 day exposure (13 ppm trans-nonachlor in feed) (Bondy et al., 2004). Collectively, these studies suggest that OC exposure may be associated with an increased risk of development of T2D and related pathologies.
Insecticides of several distinct classes may directly elevate the levels of ROS, such as superoxide radical anion and hydrogen peroxide, in cells and tissues by a variety of mechanisms. For example, inefficient oxidative metabolism of these compounds, or futile cycling leading to cytochrome P450 decoupling, is accompanied by increased levels of ROS, depletion of antioxidant defenses, and elevated lipid peroxidation (Stevenson et al., 1999; Alavanja and Bonner, 2012). Abnormal activation of NADPH oxidase (Nox), an enzyme complex responsible for the biosynthesis of superoxide radical anion, was noted during pesticide-mediated oxidative stress induction in many different cell types (Tithof et al., 2000; Abid et al., 2007; Gomez et al., 2007; Mao and Liu, 2008; Mangum et al., 2015)
Environmental factors, such as oxidative stress-inducing environmental contaminants may act as risk factors for CVD in addition to traditional risk factors. This study sought to determine whether isoprostanes, biomarkers of systemic oxidative stress possibly stemming from OC exposure, and serum concentrations of OC compounds are associated with a clinical diagnosis of atherosclerosis. Serum concentrations of p,p'-dichlorodiphenyldichloroethene (DDE) and a component and a metabolite of technical chlordane (trans-nonachlor and oxychlordane, respectively) were quantified in 200 human samples. DDE, trans-nonachlor, and oxychlordane were selected for study as these chemicals were the most frequently detected of 13 OC compounds scanned for in Mississippi soil samples (preliminary results) and previously reported to be associated with diabetes (Lee et al., 2006a). DDT, the parent compound of DDE, and chlordane were used extensively in the State in agriculture and as a termiticide, respectively. Isoprostane concentrations in matching urine samples were also determined and compared to serum OC levels and atherosclerosis disease status.
Materials and Methods
Study Sample
The study population and sample collection previously were described previously in detail (Coombes et al., 2011). Participants in the study were enrolled by convenience sampling from the clinical practice of Cardiology Associates of North Mississippi, a cardiology group in Tupelo, MS with a catchment population of approximately 250,000 from 26 counties comprising Northeast Mississippi and Northwest Alabama. All of the clinic’s patients 45 years of age and older who were to have venous access as part of their routine medical care were eligible to participate in the study. Patients with known human immunodeficiency virus or hepatitis B infection, who did not self identify as either Caucasian or African American, or who were being evaluated for unstable angina or a recent myocardial infarction were excluded. Two hundred participants (96 with atherosclerosis and 104 without atherosclerosis) were enrolled in the study with a racial composition of 120 Caucasians (60 male and 60 female) and 80 African Americans (40 male and 40 female). This demographic composition was selected to represent the general population of Mississippi.
The research division of the cardiology clinic, Cardiology Associates Research, LLC1 (CARe), enrolled the study participants. Both the Institutional Review Board of Mississippi State University and Institutional Review Board of North Mississippi Medical Center (the review board for CARe) approved the research protocol and provided oversight of the study.
Patients provided informed written consent to participate in the study. Blood samples were then collected at the time venous access was established as required by their routine medical care. The blood was allowed to clot and centrifuged at 2500–3000 × g at 4°C for 10 to 20 min. The serum was collected, frozen on dry ice, and stored at −80°C until analyzed. A spot urine sample was also collected, frozen on dry ice, and stored at −80°C until analyzed.
Participants were classified as having atherosclerosis as follows: (1) history of a clinical event (e.g. myocardial infarction or cerebrovascular accident) resulting from coronary artery disease, cerebrovascular disease, abdominoaortic disease, or peripheral vascular disease, (2) mechanical revascularization procedure (e.g. angioplasty or surgical bypass procedure) for treatment of coronary artery disease, cerebrovascular disease, abdominoaortic disease, or peripheral vascular disease, or (3) no clinical history of atherosclerosis but a reversible perfusion defect on a nuclear stress test interpreted as myocardial ischemia. Participants not meeting the criteria for atherosclerosis were considered not to have atherosclerosis. Study participants were not selected based upon their atherosclerosis status.
The clinical variables obtained for each individual from review of the patient’s clinical records and questioning of the patient are shown in Table 1. A fasting lipid panel was also obtained on each serum sample collected. All information was recorded in a data collection sheet created for this study.
Table 1.
Characterization of the Study Population
| Variables | Units | Atherosclerosis n = 96 |
Non-Atherosclerosis n = 104 |
|---|---|---|---|
| Age | Years | 64 ± 9 | 61 ± 9 |
| Gender (Male/Female) | 65/31 | 35/69 | |
| Race (African American/Caucasian) |
35/61 | 45/59 | |
| Smoker (ever/never) | 65/31 | 48/56 | |
| Diabetes (yes/no) | 35/61 | 35/69 | |
| Hypertension (yes/no) | 79/17 | 75/29 | |
| Height | Inches | 68 ± 4 | 67 ± 4 |
| Weight | Pounds | 196 ± 43 | 201 ± 46 |
| BMI | kg/m2 | 30 ± 5 | 32 ± 7 |
| HDL Cholesterol | mg/dl | 43 ± 14 | 51 ± 16 |
| LDL Cholesterol | mg/dl | 95 ± 36 | 119 ± 32 |
| Triglycerides | mg/dl | 160 ± 121 | 138 ± 67 |
| Total Cholesterol | mg/dl | 169 ± 45 | 200 ± 41 |
| Statin use (yes/no) | 80/16 | 41/63 | |
| HDL group (low/normal) | 48/47 | 28/73 | |
| LDL group (elevated/normal) |
91/5 | 65/36 | |
| Triglyceride group (normal/abnormal) |
11/25 | 52/18 | |
| Total Cholesterol group normal/abnormal |
9/18 | 28/51 | |
| DDEa | ng/gm lipid | 797 ± 1310 Nondetect=11 |
704 ± 857 Nondetect=6 |
| trans-nonachlora | ng/gm lipid | 56.8 ± 66.1 Nondetect=26 |
42.0 ± 30.3 Nondetect=30 |
| Oxychlordanea | ng/gm lipid | 10.9 ± 6.0 Nondetect=71 |
16.6 ± 22.1 Nondetect=82 |
| 8-isoPGF2αb | pmol/mg creatinine | 8.1 ± 6.3 | 7.0 ± 5.4 |
95 atherosclerosis samples and 99 non-atherosclerosis samples were available for analysis, mean and standard deviations do not include nondetectable samples
88 atherosclerosis samples and 94 non-atherosclerosis samples were available for analysis, all samples had measurable amounts of 8-isoPGF2α
Several clinical categorical variables were constructed from the clinical information obtained. Smoking status of participants was defined as “ever” for those currently smoking or who reported ever smoking and “never” for those who reported never smoking. Total cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides were all made categorical variables due to the large number of participants being treated pharmacologically for abnormal lipids. Total cholesterol was defined as high for values ≥ 200 mg/dl and normal for values < 200 mg/dl for participants not taking cholesterol lowering medicine. Participants with values < 200 mg/dl and on a statin were classified indeterminate as it was assumed statin therapy was targeted at LDL levels. LDL cholesterol was defined as normal for values ≤ 160 mg/dl, the participant did not meet criteria for drug therapy (McKenney, 2003), and the participant was not taking a statin. LDL cholesterol was classified as high for values > 160 mg/dl, the participant was taking a statin or met criteria for pharmacologic therapy for their LDL cholesterol levels and clinical situation. HDL cholesterol was defined as normal for values ≥ 40 mg/dl and low for values < 40 mg/dl. Triglycerides were defined as normal for values < 200 mg/dl for participants not taking a fibrate. Triglycerides were defined as high for values ≥ 200 mg/dl or the participant was taking a fibrate. Triglycerides were considered indeterminate for values < 200 mg/dl for participants taking a statin.
Isoprostane Analysis
Materials
Analytical standards and [2H]-stable isotope labeled standards of 8-iso-prostaglandin F2α were purchased from Cayman Chemicals (Ann Arbor, MI). Solvents for urine extractions were purchased from Burdick and Jackson (Morristown, NJ). Solid-phase extraction columns and LC-MS solvents were purchased from Thermo Fisher Scientific (Waltham, MA).
Sample Preparation
Urine samples were prepared for analysis by solid-phase extraction using Thermo HyperSep C18 and silica columns. Briefly, 1 ml urine sample was mixed with 9-ml pH 3 water and spiked with [2H4]-8-iso-PGF2α (140 pmol) internal standard. This mixture was subsequently applied to a pretreated 500 mg C-18 Sep-Pak cartridge (6 ml), followed by wash steps using 10-ml pH 3 water and 10-ml hexane. The adsorbed analytes were eluted with 10-ml ethyl acetate, dried under nitrogen (N2), and re-dissolved in 0.5 ml of ethyl acetate. This solution was applied to a pre-equilibrated silica Sep-Pak cartridge (Thermo Scientific), washed with 5-ml ethyl acetate, and subsequently eluted with 5 ml 1:1 v/v ethyl acetate/methanol. After evaporation under N2, the extract was re-suspended in 100 µl 3:1 v/v water/methanol, filtered, and placed in an LC vial insert for analysis.
UPLC-ESI MS/MS Analysis
Isoprostane concentrations were quantified using a Waters Acquity Ultra Performance Liquid Chromatograph coupled to a Thermo Scientific TSQ Quantum Access MAX triple quadrupole mass spectrometer using the method developed and validated by Masoodi and Nicolaou (2006). Chromatography was performed by injecting 10 µl each sample onto an Acquity UPLC BEH C18 column (2.1 × 50 mm, 1.7 µm) equipped with a VanGuard precolumn (2.1 × 5 mm, 1.7 µm) and eluting analytes with water(A)/methanol(B) + 0.1% acetic acid as the mobile phases using the following gradient program at a flow rate of 0.4 ml/min: 0 min (85% A, 15% B), 0.75 min (85% A, 15% B), 1.5 min (70% A, 30% B), 3.5 min (53% A, 47% B), 5 min (46% A, 54% B), 6 min (45% A, 55% B), 10.5 min (40% A, 60% B), 15 min (30% A, 70% B), 16 min (20% A, 80% B), 17 min (0% A, 100% B), 19 min (0% A, 100% B), 19.5 min (85% A, 15% B) and 21 min (85% A, 15% B). ESI-MS/MS analysis of a selected 15-series F2-isoprostane, 8-iso-PGF2α, by single reaction monitoring (SRM) with heated electrospray ionization (HESI) was accomplished by monitoring the m/z 353>193 transition throughout the chromatographic run. The internal standard [2H4]-8-iso-PGF2α was monitored by the transition m/z 357>197. 8-iso-PGF2α concentrations were calculated using the following equation:
Analyte concentration = (analyte area / ionization efficiency correction factor) × (internal standard concentration / internal standard area). Isoprostane levels were standardized to urinary creatinine in all samples (Viau et al., 2004).
Creatinine Standardization
Urinary creatinine concentrations were measured using a Diagnostic Chemicals Limited Creatinine-S kit using a protocol optimized for absorbance measurement using a BioTek Synergy spectrophotometer at 490 nm.
Organochlorine Compound Analysis
Materials
Analytical standards and [13C]-stable isotope labeled standards of DDE, trans-nonachlor, and oxychlordane were purchased from ChemService, Inc (West Chester, PA). Solvents for serum extractions were purchased from Thermo Fisher Scientific (Waltham, MA). Solid-phase extraction columns were purchased from DPX Labs (Columbia, SC).
Sample Preparation
Serum OC compounds were extracted using DPX Labs reverse-phase extraction columns as previously described in Eden et al. (2014). Briefly, 1 ml serum (stored frozen at −80°C) was thawed and 100 µl internal standard solution containing [13C]-p,p’-DDT and [13C]-trans-nonachlor in hexane (final concentrations, 0.01 µg/ml) was added to each sample. Plasma samples were vortexed for 1 min followed by a 2 ml addition of acetonitrile to deproteinize samples. Samples were subsequently centrifuged at 3500g for 10 min and supernatant was collected in a separate tube. Deionized water (2 ml) was added and each sample was then aspirated into an individual DPX column for 30 sec and supernatant discarded. Columns were washed by aspirating 0.5 ml of 33% (v/v) acetonitrile in water for 10 sec and the wash solution discarded. The OC compounds were collected through 2 elution steps; first a 1 ml aliquot of elution solvent (50/50 v/v ethyl acetate/hexane) was added to the top of the column and forced through the sorbent material, followed by an additional 0.5 ml of elution solvent. Extracted samples were dried under a N2 stream and finally re-suspended in 100 µl of 1:1 v/v ethyl acetate/hexane for analysis.
GC-MS Analysis
Serum concentrations of three OC compounds DDE, trans-nonachlor, and oxychlordane were quantified as previously described (Eden et al., 2014). Briefly, extracted samples were assayed via gas chromatography-mass spectrometry (GC-MS) on an Agilent Technologies 6890N GC and autosampler coupled to an Agilent Technologies 5975C Triple Axis MS Detector employing electron ionization. Peak integration of the individual extracted ion chromatogram for each analyte of interest was performed using an automated integration program utilizing both qualitative and quantitative ions (Barr et al., 2003; Eden et al., 2014). Standard curve data were established for each analyte using purchased pooled “blank” human serum that did not contain detectable levels of OC compounds. Analyte concentrations were adjusted for serum lipid concentration (calculated from total cholesterol and triglyceride data) as described in the analysis performed in the 1999–2000 NHANES study (CDC, 2009) and expressed in ng analyte/g lipid. Samples with OC concentrations below the limit of detection (LOD) (100 pg/ml serum for all analytes) were assigned a value of 0 ng/g lipid.
Statistical Analysis
Statistical analysis was performed using SAS for Windows version 9.3 (SAS Institute Inc., Cary, NC). The association of serum OC concentrations with urinary isoprostane levels, regardless of disease status or other clinical and demographic factors, was analyzed by linear regression using PROC REG. Possible associations between atherosclerosis and explanatory variables, including urinary isoprostane concentrations and serum OC levels, were analyzed via univariable logistic regression using PROC LOGISTIC. Because only 42 of 196 analyzed subjects possessed detectable levels of oxychlordane, serum concentrations of this compound were analyzed as a dichotomous variable, i.e. present/detectable vs. absent/ non-detectable, for the purposes of logistic regression analysis.
Multivariable logistic regression modeling was subsequently used to assess the association of atherosclerosis occurrence with clinical factors, serum OC concentrations, and urinary isoprostane levels. Using only clinical and demographic factors, a core multivariable logistic regression model for atherosclerosis was previously constructed for this study sample (Coombes et al., 2011). Total cholesterol and triglyceride groups were excluded as candidate explanatory variables due to the large number of individuals indeterminate due to drug therapy. Briefly, the strength of association of atherosclerosis with each of the candidate explanatory variables (clinical factors) was assessed by logistic regression (PROC LOGISTIC; SAS for Windows v. 9.3). Clinical factors associated with atherosclerosis with a P value ≤ 0.25 were used as candidate explanatory variables in the construction of the core multivariable model at α = 0.1. The model was fit to the candidate explanatory variables and the explanatory variable with the highest P value > 0.1 was removed and the model was refit to the remaining explanatory variables. This manual backward selection stepwise process was continued until only explanatory variables with P ≤ 0.1 remained.
8-iso-PGF2α levels and serum OC concentrations were then added both individually and together as a group to the core multivariable model as both main effects and as two-way interaction terms with all other variables in the core model to assess their association with atherosclerosis. Urinary isoprostanes and all serum OC values were included in this analysis, regardless of the strength of association with atherosclerosis previously determined by univariable logistic regression analysis. Prior to inclusion in the multivariable model, collinearity among potential explanatory variables was assessed by Spearman rank correlation using PROC CORR. Categorical variables were assigned values of 0 (absent/no) or 1 (present/yes) for this analysis. The maximum allowable Spearman Correlation Coefficient of any two explanatory variables to be included in the same model was set at 0.8. If two variables were found to be collinear, a judgment was made as to which of the two variables to retain as an objective predictor of atherosclerosis. The multivariable model was then constructed using the previously described manual backward selection process. If the variable exhibiting the largest P value at a given selection step was a main effect that was also represented as an interaction term with P ≤ 0.1, both the main effect and interaction term were retained in the model.
Results
Study Sample Characteristics
Clinical, demographic, serum levels of DDE, trans-nonachlor, and oxychlordane, and urinary isoprostane levels of participants in the study are presented in Table 1. Surprisingly, LDL cholesterol was noted to be higher in individuals without atherosclerosis. However, cholesterol therapy guidelines mandate more aggressive treatment of individuals with known disease, and in fact patients with atherosclerosis were more likely to be on statin therapy probably accounting for this finding. Due to the large number of individuals receiving therapy for dyslipidemia (statins and fibrates), categorical variables were constructed from serum levels of HDL cholesterol, LDL cholesterol, triglycerides, total cholesterol, and current medications for further analysis. DDE was the most abundant OC compound of the 3 compounds in this study sample while oxychlordane was the least abundant. These observations are consistent with average serum OC concentrations reported previously for North American and European populations (Eden et al., 2014; Glynn et al., 2003; Min et al., 2011). Isoprostanes were detectable in all urine samples and creatinine-adjusted concentrations varied considerably within the study sample.
Association of urinary 8-iso-PGF2α with serum OC concentrations
Linear regression analysis was used to assess any association of urinary isoprostane levels with serum concentrations of each OC compound individually. No significant association was found between urinary isoprostane concentrations and serum DDE, trans-nonachlor, or oxychlordane levels. Of note, the non-detect samples from each of the OC compounds needed to be excluded from the analysis as their inclusion resulted in a non-normal distribution of residuals, a violation of a required assumption for linear regression.
Univariable Logistic Regression Analysis
Possible associations between atherosclerosis and urinary isoprostane and serum OC levels were determined by univariable logistic regression (Table 2). Urinary isoprostane levels and serum levels of DDE and trans-nonachlor were analyzed as continuous variables while oxychlordane was analyzed as a dichotomous variable due to the large number of samples with no detectable levels of the pesticide (154 of 200). In this study population, urinary isoprostane levels were not significantly associated with atherosclerosis. None of the lipid corrected serum levels of OC compounds were found to be markedly associated with atherosclerosis. However, trans-nonachlor was noted to fall just short of the threshold set for statistical significance.
Table 2.
Univariable Logistic Regression Analysis of the Association of Atherosclerosis with Urinary Isoprostane and Serum OC Variables
| Factor | Comparison | Units | n | Odds Ratio | 95% CI | P value |
|---|---|---|---|---|---|---|
| 8-iso-PGF2α | Increasing | 1 pmol/mg creatinine |
182 | 1.03 | 0.98 – 1.08 | 0.241 |
| trans-nonachlor | Increasing | 10 ng/g lipid | 196 | 1.06 | 0.99– 1.13 | 0.102 |
| p,p'-DDE | Increasing | 10 ng/g lipid | 196 | 1.00 | 1.00 – 1.01 | 0.775 |
| Oxychlordane | Present vs Absent |
196 | 1.63 | 0.81 – 3.28 | 0.170 |
Multivariable Logistic Regression Modeling
Prior to the creation of a multivariable logistic regression model for atherosclerosis in this study sample, collinearity among all potential explanatory variables, including clinical factors, serum OC levels, and urinary isoprostane levels, was assessed by Spearman rank correlation. No collinearity was observed between any of the explanatory variables. Multivariable logistic regression models were then constructed to assess the association of clinical factors, serum concentrations of DDE and trans-nonachlor, presence versus absence of oxychlordane, and urinary isoprostane levels with atherosclerosis. A core atherosclerosis model was constructed using only clinical explanatory variables as described in materials and methods and reported in Coombes et al. (2011) and shown in Table 3. This core model contains gender, age, smoking history, LDL group, and hypertension as explanatory variables. Each of the serum OC explanatory variables and urinary isoprostane concentration were then added individually to the core model. The resulting model containing serum trans-nonachlor concentration is presented in Table 4. Addition of trans-nonachlor to the core atherosclerosis model (retaining all clinical factors) increased the maximum rescaled generalized r2 value. When this model was refit, hypertension was removed and the final model is shown in Table 5. Serum trans-nonachlor concentration was retained in this model and is significantly associated with atherosclerosis though the maximum rescaled generalized r2 value decreased slightly from the model containing hypertension (Table 4) but was still larger than the r2 of the core model (Table 3). Addition of DDE, oxychlordane, or urinary isoprostanes individually to the core model and subsequent refitting of the model resulted in each of these variables being removed due to a lack of statistical significance. If all the serum OC concentrations were added to the core model as a group with stepwise refitting of the model, only trans-nonachlor was retained in the final model which was identical to the model in Table 5. Thus, the analysis showed only trans-nonachlor was associated with atherosclerosis.
Table 3.
Core Multivariable Logistic Regression Model of Clinical Variables Associated with Atherosclerosis Occurrence (adapted from Coombes et al. 2011)
| Max-rescaled r2 = 0.35, AIC=226, Hosmer and Lemeshow Goodness-of-Fit test P =0.177 (n= 197) | |||||
|---|---|---|---|---|---|
| Factor | Comparison | Units | Odds Ratio | 95% CI | P value |
| Gender | Male vs. female | 3.26 | 1.66 – 6.42 | <0.001 | |
| Age | Increasing | 5 years | 1.05 | 1.01 – 1.09 | 0.011 |
| Smoking History | Ever vs. never | 2.35 | 1.19 – 4.63 | 0.014 | |
| LDL group | High vs. normal | 7.27 | 2.55 – 20.76 | <0.001 | |
| Hypertension | Yes vs. no | 2.16 | 0.96 – 4.87 | 0.063 | |
Abbreviations: LDL, low-density lipoprotein; AIC, Akaike Information Criterion
Table 4.
Multivariable Logistic Regression Model of Clinical Variables and Serum trans-Nonachlor Levels Associated with Atherosclerosis Occurrence
| Max-rescaled r2 =0.37, AIC=217, Hosmer and Lemeshow Goodness-of-Fit test P =0.173 (n= 191) | |||||
|---|---|---|---|---|---|
| Factor | Comparison | Units | Odds Ratio | 95% CI | P value |
| Gender | Male vs. female | 3.27 | 1.62 – 6.57 | <0.001 | |
| Age | Increasing | 5 years | 1.05 | 1.01 – 1.09 | 0.028 |
| Smoking History | Ever vs. never | 2.78 | 1.37 – 5.65 | 0.005 | |
| LDL group | High vs. normal | 7.29 | 2.52 – 21.11 | <0.001 | |
| Hypertension | Yes vs. no | 1.86 | 0.80 – 4.32 | 0.147 | |
| trans-nonachlor | Increasing | 10 ng/g lipid | 1.08 | 0.99 – 1.18 | 0.103 |
Abbreviations: LDL, low-density lipoprotein; AIC, Akaike Information Criterion
Table 5.
Alternative Multivariable Logistic Regression Model of Clinical Variables and Serum trans-Nonachlor Levels Associated with Atherosclerosis Occurrence (without Hypertension)
| Max-rescaled r2 = 0.36, AIC=218, Hosmer and Lemeshow Goodness-of-Fit test p =0.147 (n= 191) | |||||
|---|---|---|---|---|---|
| Factor | Comparison | Units | Odds Ratio | 95% CI | P value |
| Gender | Male vs. female | 3.19 | 1.60 – 6.39 | 0.001 | |
| Age | Increasing | 5 years | 1.04 | 1.00 – 1.08 | 0.043 |
| Smoking History | Ever vs. never | 2.68 | 1.33 – 5.39 | 0.006 | |
| LDL group | High vs. normal | 7.91 | 2.74 – 22.77 | <0.001 | |
| trans-nonachlor | Increasing | 10 ng/g lipid | 1.09 | 1.00 – 1.19 | 0.074 |
Abbreviations: LDL, low-density lipoprotein; AIC, Akaike Information Criterion
Discussion
The present study sought to assess the association of OC compound exposure (serum OC levels) with systemic oxidative stress (urinary isoprostane levels). Although no statistically significant association was found between urinary 8-iso-PGF2α levels and serum concentrations of DDE, trans-nonachlor, or oxychlordane in this study sample, serum trans-nonachlor was independently associated with a clinical diagnosis of atherosclerosis in a multivariable logistic regression model of atherosclerosis. Addition of serum trans-nonachlor to the core multivariable atherosclerosis model originally described in Coombes et al. (2011) (Table 5) resulted in improvement in the model’s explanatory power although hypertension was no longer markedly associated with atherosclerosis (Table 4: r2 = 0.37; hypertension P value = 0.147). When this model was refit, an alternative model was generated no longer containing hypertension but containing trans-nonachlor (Table 5: trans-nonachlor P value = 0.074). This model still demonstrated a small improvement from the core model’s explanatory power (Table 5: r2 = 0.36 versus Table 3: r2 = 0.35). Thus a statistically significant association of serum trans-nonachlor with clinical atherosclerosis was noted in this study sample. This result is consistent with previous epidemiological evidence that trans-nonachlor and related OC compounds are risk factors for cardiovascular disease. In a subpopulation of obese subjects from a 1999–2004 NHANES dataset, Min et al. (2011) reported a significant association between peripheral arterial disease, a form of CVD, and circulating levels of DDE (OR=1.47), trans-nonachlor (OR=1.68), oxychlordane (OR=1.82) and dieldrin (OR=2.36). A similar study conducted by Ha et al. (2007) noted a significant positive association between serum OC compound levels (summed concentrations of DDE, oxychlordane, trans-nonachlor and heptachlor epoxide) and self-reported CVD in females in a 1999–2002 NHANES dataset. In addition, a recent cross-sectional investigation of possible associations between CVD and POP content of human lipoprotein fractions by Ljunggren et al. (2014) demonstrated significantly elevated concentrations of trans-nonachlor and various PCB congeners in the low-density lipoprotein/very low-density lipoprotein fractions of individuals with CVD compared to healthy controls, a finding of potential importance to understanding the physiological basis of an association between persistent OC body burden and CVD.
The lack of a significant association between elevated urinary isoprostanes and a diagnosis of atherosclerosis in this study is inconsistent with previous observations indicating that isoprostanes are a useful biomarker of systemic oxidative stress that is linked to CVD status (Vassalle et al., 2003; Schwedhelm et al., 2004; Kim et al., 2012; Menazza et al., 2014). This finding may be attributable to the makeup of the particular study sample examined in this project. The sample size was small and sampling was by convenience. Another potential factor that complicated efforts to examine the relationship between atherosclerosis and urinary isoprostanes and serum OC variables was the patient group without atherosclerosis in this study (the control group) was biased in that these individuals were referred to a cardiology clinic for evaluation and/or treatment of potential CVD. The abnormally high prevalence of T2D in this group is evidence that these individuals are, in general, in poorer health than a truly random group of people from the general population of similar age, race and gender. This possibility is further supported by our observations that urinary isoprostane excretion levels for non-atherosclerotic (control) individuals in this study sample were higher, on average, than corresponding values reported for healthy individuals in the literature. Previous studies reported mean urinary 8-iso-PGF2α concentrations for healthy subjects ranging from 0.68 to 3.23 pmol/mg creatinine (Mori et al., 1999 ; Schwedhelm et al., 2004; Kim et al., 2012). In contrast, the mean urinary isoprostane level for the control group in the current study was 7.04 pmol/mg creatinine. Any potential association between urinary isoprostane concentrations and circulating OC compound levels or atherosclerosis in this study sample may have been masked by over-representation of other pathophysiological processes that contribute to overall systemic oxidative stress, such as obesity and T2D (Morrow, 2005; Milne et al., 2007; Montine et al., 2011; Coombes et al., 2011).
A growing body of evidence suggests that exposure to environmental pollutants, including OC such as trans-nonachlor and DDE, may predispose a population initially free of disease to the onset of atherosclerosis, dyslipidemia, obesity, and impaired glucose tolerance (Lee et al., 2011; Min et al., 2011). Therefore, it is imperative to investigate the potential of these compounds to induce and/or exacerbate these pathologies and assess whether they can be used to identify at-risk patients. Although the oxidative stress hypothesis of xenobiotic-induced disease is attractive, several unanswered questions remain. It is difficult to determine whether oxidative stress biomarkers arise from the direct effects of pesticides in cells, or result instead from maladaptive responses to stressors and damaged cells. Moreover, many disease states, such as cancer, obesity, T2D, neurodegenerative disorders and atherosclerosis induce oxidative stress biomarkers in blood and urine. Identification of biomarkers that distinguish between oxidative stress resulting from specific chemical exposures, such as pesticides, versus those stemming from other disease processes are needed and would greatly benefit this field of study.
In conclusion, these data demonstrated an association between serum trans-nonachlor levels and clinical atherosclerosis. However, a significant association between urinary isprostane concentrations, a biomarker of systemic oxidative stress, and atherosclerosis outcomes was not found. The study population examined was unique in that it was centered in the Deep South of the U.S. (Tupelo, Mississippi) and had an approximate 60/40% racial split of Caucasians and African-Americans. Thus, it is highly representative of the population found in Mississippi and other neighboring states where CVD is particularly prevalent. This lends innovation to the study because this particular population has been understudied with regard to etiologic factors contributing to disease outcomes.
Acknowledgments
Funding
The project described was supported by Award Number R21ES015107 from the National Institute of Environmental Health Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Environmental Health Sciences or the National Institutes of Health.
Footnotes
Director: Dr. James C. Johnson Jr and Manager: Marsha R. Jones
References
- Abid MR, Spokes KC, Shih SC, Aird WC. NADPH oxidase activity selectively modulates vascular endothelial growth factor signaling pathways. J. Biol. Chem. 2007;282:35373–35385. doi: 10.1074/jbc.M702175200. [DOI] [PubMed] [Google Scholar]
- Alavanja MC, Bonner MR. Occupational pesticide exposures and cancer risk: A review. J. Toxicol. Environ. Health B. 2012;15:238–263. doi: 10.1080/10937404.2012.632358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr JR, Maggio VL, Barr DB, Turner WE, Sjodin A, Sandau JL, Pirkle JL, Needham LL, Patterson DG., Jr New high-resolution mass spectromatic approach for the measurement of polychlorinated biphenyls and organochlorine pesticides in human serum. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2003;794:137–148. doi: 10.1016/s1570-0232(03)00451-3. [DOI] [PubMed] [Google Scholar]
- Bondy G, Curran I, Doucet J, Armstrong C, Coady L, Hierlihy L, Fernie S, Robertson P, Barker M. Toxicity of trans-nonachlor to Sprague–Dawley rats in a 90-day feeding study. Food Chem. Toxicol. 2004;42:1015–1027. doi: 10.1016/j.fct.2004.02.014. [DOI] [PubMed] [Google Scholar]
- CDC. Fourth national report on human exposure to environmental chemicals. Atlanta (GA): Centers for Disease Control and Prevention; 2009. [Google Scholar]
- Coombes RH, Crow JA, Dail MB, Chambers HW, Wills RW, Bertolet BD, Chambers JE. Relationship of human paraoxonase-1 serum activity and genotype with atherosclerosis in individuals from the Deep South. Pharmacogenet. Genom. 2011;21:867–875. doi: 10.1097/FPC.0b013e32834cebc6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davi G, Falco A, Patrono C. Determinants of F2-isoprostane biosynthesis and inhibition in man. Chem. Phys. Lipids. 2004;128:149–163. doi: 10.1016/j.chemphyslip.2003.10.001. [DOI] [PubMed] [Google Scholar]
- de Broucker V, Hulo S, Cherot-Kornobis N, Sobaszek A, Edme JL. Increased levels of 8-isoprostane in EBC of NO2-exposed rats. J. Toxicol. Environ. Health A. 2015;78:666–670. doi: 10.1080/15287394.2015.1023915. [DOI] [PubMed] [Google Scholar]
- Eden PR, Meek EC, Wills RW, Olsen EV, Crow JA, Chambers JE. Association of type 2 diabetes mellitus with plasma organochlorine compound concentrations. J. Expo. Sci. Environ. Epidemiol. 2014;22:69–75. doi: 10.1038/jes.2014.69. [DOI] [PubMed] [Google Scholar]
- Glynn AW, Granath F, Aune M, Atuma S, Darnerud PO, Bjerselius R, Vainio H, Weiderpass E. Organochlorines in Swedish women: Determinants of serum concentrations. Environ. Health. Persp. 2003;111:349–355. doi: 10.1289/ehp.5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gniwotta C, Morrow JD, Roberts LJ, 2nd, Kuhn H. Prostaglandin F2-like compounds, F2-isoprostanes, are present in increased amounts in human atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 1997;17:3236–3241. doi: 10.1161/01.atv.17.11.3236. [DOI] [PubMed] [Google Scholar]
- Gomez C, Bandez MJ, Navarro A. Pesticides and impairment of mitochondrial function in relation with the parkinsonian syndrome. Front. Biosci. 2007;12:1079–1093. doi: 10.2741/2128. [DOI] [PubMed] [Google Scholar]
- Ha MH, Lee DH, Jacobs DR. Association between serum concentrations of persistent organic pollutants and self-reported cardiovascular disease prevalence: Results from the National Health and Nutrition Examination Survey, 1999–2002. Environ. Health. Persp. 2007;115:1204–1209. doi: 10.1289/ehp.10184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadiiska MB, Gladen BC, Baird DD, Germolec D, Graham LB, Parker CE, Nyska A, Wachsman JT, Ames BN, Basu S, Brot N, Fitzgerald GA, Floyd RA, George M, Heinecke JW, Hatch GE, Hensley K, Lawson JA, Marnett LJ, Morrow JD, Murray DM, Plastaras J, Roberts LJ, 2nd, Rokach J, Shigenaga MK, Sohal RS, Sun J, Tice RR, Van Thiel DH, Wellner D, Walter PB, Tomer KB, Mason RP, Barrett JC. Biomarkers of oxidative stress study II: Are oxidation products of lipids, proteins, and DNA markers of CCl4 poisoning? Free Radic. Biol. Med. 2005;38:698–710. doi: 10.1016/j.freeradbiomed.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Kharasch ED, Hankins DC, Fenstamaker K, Cox K. Human halothane metabolism, lipid peroxidation, and cytochromes P(450)2A6 and P(450)3A4. Eur. J. Clin. Pharmacol. 2000;55:853–859. doi: 10.1007/s002280050707. [DOI] [PubMed] [Google Scholar]
- Kim J-Y, Lee J-W, Youn Y-J, Ahn M-S, Ahn S-G, Yoo B-S, Lee S-H, Yoon J, Choe K-H. Urinary levels of 8-iso-prostaglandin F2α and 8-hydroxydeoxyguanine as markers of oxidative stress in patients with coronary artery disease. Korean Circ. J. 2012;42:614–617. doi: 10.4070/kcj.2012.42.9.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DH, Lee IK, Song K, Steffes M, Toscano W, Baker BA, Jacobs DR., Jr A strong dose-response relation between serum concentrations of persistent organic pollutants and diabetes: Results from the National Health and Examination Survey 1999–2002. Diabetes Care. 2006a;29:1638–1644. doi: 10.2337/dc06-0543. [DOI] [PubMed] [Google Scholar]
- Lee DH, Jacobs DR, Porta M. Could low-level background exposure to persistent organic pollutants contribute to the social burden of type 2 diabetes? J. Epidemiol. Community Health. 2006b;60:1006–1008. doi: 10.1136/jech.2006.053389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D-H, Steffes MW, Sjödin S, Jones RS, Needham LL, Jacobs DR., Jr Low dose organochlorine pesticides and polychlorinated biphenyls predict obesity, dyslipidemia, and insulin resistance among people free of diabetes. PLoS ONE. 2011;6:e15977. doi: 10.1371/journal.pone.0015977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitinger N, Huber J, Rizza C, Mechtcheriakova D, Bochkov V, Koshelnick Y, Berliner JA, Binder BR. The isoprostane 8-iso-PGF(2alpha) stimulates endothelial cells to bind monocytes: Differences from thromboxane-mediated endothelial activation. FASEB J. 2001;15:1254–1256. doi: 10.1096/fj.00-0498fje. [DOI] [PubMed] [Google Scholar]
- Ljunggren SA, Helmfrid I, Salihovic S, van Bavel B, Wingren G, Lindahl M, Karlsson H. Persistent organic pollutants distribution in lipoprotein fractions in relation to cardiovascular disease and cancer. Environ. Int. 2014;65:93–99. doi: 10.1016/j.envint.2013.12.017. [DOI] [PubMed] [Google Scholar]
- Longnecker MP, Daniels JL. Environmental contaminants as etiologic factors for diabetes. Environ. Health Persp. 2001;109:871–876. doi: 10.1289/ehp.01109s6871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangum LC, Borazjani A, Stokes JV, Matthews AT, Lee JH, Chambers JE , Ross MK. Organochlorine insecticides induce NADPH oxidase-dependent reactive oxygen species in human monocytic cells via phospholipase A2/arachidonic acid. Chem. Res. Toxicol. 2015;28:570–584. doi: 10.1021/tx500323h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao H, Liu B. Synergistic microglial reactive oxygen species generation induced by pesticides lindane and dieldrin. Neuroreport. 2008;19:1317–1320. doi: 10.1097/WNR.0b013e32830b3677. [DOI] [PubMed] [Google Scholar]
- Masoodi M, Nicolaou A. Lipidomic analysis of twenty-seven prostanoids and isoprostanes by liquid chromatography/electrospray tandem mass spectrometry. Rapid Commun. Mass Spectrometry. 2006;20:3023–3029. doi: 10.1002/rcm.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenney JM. Update on the National Cholesterol Education Program adult treatment panel III: Getting to goal. Pharmacotherapy. 2003;23:26S–33S. doi: 10.1592/phco.23.11.26s.32710. [DOI] [PubMed] [Google Scholar]
- Menazza S, Canton M, Sorato E, Boengler K, Schulz R, Di Lisa F. Old and new biomarkers of oxidative stress in heart failure. Drug Discov. Today: Ther. Strateg. 2012;9:e189–e198. [Google Scholar]
- Milne GL, Yin H, Brooks JD, Sanchez S, Roberts LJ, 2nd, Morrow JD. Quantification of F2-isoprostanes in biological fluids and tissues as a measure of oxidant stress. Meth. Enzymol. 2007;433:113–126. doi: 10.1016/S0076-6879(07)33006-1. [DOI] [PubMed] [Google Scholar]
- Milne GL, Yin H, Hardy KD, Davies SS, Roberts LJ., 2nd Isoprostane generation and function. Chem. Rev. 2011;111:5973–5996. doi: 10.1021/cr200160h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min JY, Cho JS, Lee KJ, Park JB, Park SG, Kim JY, Min KB. Potential role for organochlorine pesticides in the prevalence of peripheral arterial diseases in obese persons: Results from the National Health and Nutrition Examination Survey 1999–2004. Atherosclerosis. 2011;218:200–206. doi: 10.1016/j.atherosclerosis.2011.04.044. [DOI] [PubMed] [Google Scholar]
- Montine TJ, Peskind ER, Quinn JF, Wilson AM, Montine KS, Galasko D. Increased cerebrospinal fluid F2-isoprostanes are associated with aging and latent Alzheimer's disease as identified by biomarkers. Neuromolecular Med. 2011;13:37–43. doi: 10.1007/s12017-010-8126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori TA, Croft KD, Puddey IB, Beilin LJ. An improved method for the measurement of urinary and plasma F2-isoprostanes using gas chromatography-mass spectrometry. Anal. Biochem. 1999;268:117–125. doi: 10.1006/abio.1998.3037. [DOI] [PubMed] [Google Scholar]
- Morrow JD. Quantification of isoprostanes as indices of oxidant stress and the risk of atherosclerosis in humans. Arterioscler. Thromb. Vasc. Biol. 2005;25:279–286. doi: 10.1161/01.ATV.0000152605.64964.c0. [DOI] [PubMed] [Google Scholar]
- Morrow JD, Awad JA, Kato T, Takahashi K, Badr KF, Roberts LJ, 2nd, Burk RF. Formation of novel non-cyclooxygenase-derived prostanoids (F2-isoprostanes) in carbon tetrachloride hepatotoxicity. An animal model of lipid peroxidation. J. Clin. Invest. 1992;90:2502–2507. doi: 10.1172/JCI116143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow JD, Frei B, Longmire AW, Gaziano JM, Lynch SM, Shyr Y, Strauss WE, Oates JA, Roberts LJ., 2nd Increase in circulating products of lipid peroxidation (F2-isoprostanes) in smokers. Smoking as a cause of oxidative damage. N. Engl. J. Med. 1995;332:1198–1203. doi: 10.1056/NEJM199505043321804. [DOI] [PubMed] [Google Scholar]
- Pratico D, Tangirala RK, Rader DJ, Rokach J, FitzGerald GA. Vitamin E suppresses isoprostane generation in vivo and reduces atherosclerosis in ApoE-deficient mice. Nat. Med. 1998;4:1189–1192. doi: 10.1038/2685. [DOI] [PubMed] [Google Scholar]
- Roberts LJ, Morrow JD. Measurement of F(2)-isoprostanes as an index of oxidative stress in vivo. Free Radic. Biol. Med. 2000;28:505–513. doi: 10.1016/s0891-5849(99)00264-6. [DOI] [PubMed] [Google Scholar]
- Ruzzin J, Petersen R, Meugnier E, Madsen L, Lock EJ, Lillefosse H, Ma T, Pesenti S, Sonne SB, Marstrand TT, Malde MK, Du ZY, Chavey C, Fajas L, Lundebye AK, Brand CL, Vidal H, Kristiansen K, Froyland L. Persistent organic pollutant exposure leads to insulin resistance syndrome. Environ. Health Persp. 2010;118:465–471. doi: 10.1289/ehp.0901321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwedhelm E, Bartling A, Lenzen H, Tsikas D, Maas R, Brummer J, Gutzki FM, Berger J, Frolich JC, Boger RH. Urinary 8-iso-prostaglandin F2alpha as a risk marker in patients with coronary heart disease: A matched case-control study. Circulation. 2004;109:843–848. doi: 10.1161/01.CIR.0000116761.93647.30. [DOI] [PubMed] [Google Scholar]
- Stevenson DE, Walborg EF, Jr, North DW, Sielken RL, Jr, Ross CE, Wright AS, Xu Y, Kamendulis LM, Klaunig JE. Monograph: Reassessment of human cancer risk of aldrin/dieldrin. Toxicol. Lett. 1999;109:123–186. doi: 10.1016/s0378-4274(99)00132-0. [DOI] [PubMed] [Google Scholar]
- Tithof PK, Olivero J, Ruehle K, Ganey PE. Activation of neutrophil calcium-dependent and -independent phospholipases A2 by organochlorine compounds. Toxicol. Sci. 2000;53:40–47. doi: 10.1093/toxsci/53.1.40. [DOI] [PubMed] [Google Scholar]
- Vassalle C, Botto N, Andreassi MG, Berti S, Biagini A. Evidence for enhanced 8-isoprostane plasma levels, as index of oxidative stress in vivo, in patients with coronary artery disease. Coron. Artery Dis. 2003;14:213–218. doi: 10.1097/01.mca.0000063504.13456.c3. [DOI] [PubMed] [Google Scholar]
- Viau C, Lafontaine M, Payan JP. Creatinine normalization in biological monitoring revisited: The case of 1-hydroxypyrene. Int. Arch. Occup. Environ. Health. 2004;77:177–185. doi: 10.1007/s00420-003-0495-9. [DOI] [PubMed] [Google Scholar]
- Wang CX, Xu SQ, Lv ZQ, Li YY, Wang YJ, Chen T. Exposure to persistent organic pollutants as potential risk factors for developing diabetes. Sci. China-Chem. 2010;53:980–994. [Google Scholar]