Abstract
Breast cancer surgery is known to cause severe acute postoperative pain, which can persist for a long time. We administered nefopam preventively to patients undergoing lumpectomy with axillary lymph node dissection or sentinel lymph node biopsy, and evaluated its efficacy on acute and chronic postoperative pain.
Enrolled patients were assigned to the nefopam (n = 41) or the control (n = 42) group. Before initiating the operation, 20 mg of nefopam was given to the patients of the nefopam group, and normal saline was used in the control group. Ketorolac was given at the end of surgery, and meloxicam was prescribed in the postoperative period to all patients in both groups. Pain was assessed using a numerical rating scale (NRS), and the rescue analgesic drug was given when the NRS was >5. Implementation of postoperative chemotherapy, radiotherapy (RT), or hormone therapy was evaluated.
The NRS of postoperative pain was significantly lower in the nefopam than in the control group in the postanesthetic care unit (4.5 ± 2.2 vs 5.7 ± 1.5, respectively; P = 0.01), at postoperative 6 h (3.0 ± 1.6 vs 4.5 ± 1.3, respectively; P < 0.001), and at postoperative 24 h (3.1 ± 1.1 vs 3.8 ± 1.5, respectively; P = 0.01) with reduced use of rescue analgesic drugs. Significantly fewer patients suffered from chronic postoperative pain in the nefopam than in the control group at postoperative 3 months (36.6% vs 59.5%, P = 0.04). Considering only the cohort without postoperative adjuvant RT, the difference in the proportion of patients reporting chronic pain increased (23.5% in the nefopam group vs 61.5% in the control group, P = 0.04).
Preventive nefopam was helpful in reducing the acute postoperative pain, with reduced use of rescue analgesic drugs, and it contributed to reduced occurrence of chronic pain at postoperative 3 months after breast cancer surgery.
INTRODUCTION
Breast surgery is known to cause severe acute postoperative pain in more than 50% of patients, and about 8% of those patients suffer from persistent severe pain.1 Acute postoperative pain evolves into chronic pain in some surgeries. In breast surgery, moderate to severe acute postoperative pain is 1 of the predictive factors for chronic pain.2 In thoracic surgery, patients who had experienced severe acute postoperative pain after thoracotomy also reported chronic pain.3 Chronic postoperative pain may lead to functional impairment and a decline in the quality of life over time.
Several analgesic methods have been evaluated in various forms of surgery, and regional analgesia or several pharmacological agents, such as nonsteroidal anti-inflammatory drugs (NSAIDs), N-methyl-d-aspartate (NMDA) receptor antagonists, α-2-receptor agonists, and so on, are known to be helpful in postoperative pain control by attenuating central and peripheral sensitization.4
Nefopam has been studied in several animal models and various clinical settings, where it was found to be an effective analgesic adjuvant.5 Perioperative use of nefopam reduced the postoperative consumption of morphine, with surgical pain relief after abdominal and orthopedic surgeries,6,7 and it enhanced the analgesic effect of NSAIDs.8 However, to our knowledge, no reported clinical study has evaluated the analgesic efficacy of nefopam in breast cancer surgery. Furthermore, insufficient studies have examined whether and how nefopam affects the chronicity of postoperative pain. Whereas nefopam decreased both acute and chronic pain in animal models,9,10 a clinical result indicated that nefopam had little effect on chronic pain after total knee arthroplasty.11 Although the exact mechanism of nefopam is unknown, it has properties of monoamine reuptake inhibitor and NMDA receptor antagonist,12,13 which are well known to be involved in managing chronic pain.14,15
Thus, under the hypothesis that nefopam given prior to surgery as a preventive analgesic would alleviate not only acute but also chronic postoperative pain, we evaluated the analgesic efficacy of nefopam on postoperative pain associated with breast cancer surgery.
MATERIALS AND METHODS
This prospective, randomized, and controlled study was approved by the Institutional Review Board of Seoul National University Bundang Hospital (B-1503/292-008) and registered at ClinicalTrials.gov. (NCT02561468). Female patients scheduled to undergo lumpectomy with axillary lymph nodes dissection (ALND) or sentinel lymph node biopsy (SLNB) were first recruited. Their American Society of Anesthesiologist physical status was limited to I or II. Excluded patients were as follows: refusal to participate, medical history of seizure, myocardial infarction, urinary tract disease causing urinary retention, and angle-closure glaucoma, current medication with monoamine oxidase inhibitors or any kinds of analgesic drug, pregnancy or lactation, and reoperations due to the existence of cancer cells on the resection margin or cancer recurrence after a previous breast cancer surgery. Each eligible patient had signed the written informed consent before participating in this clinical study.
One anesthesiologist who did not take anesthetic care of patients was in charge of the randomization and the preparation of the nefopam. According to the computer-generated randomization table, each patient was assigned to either the nefopam group or the control group. The anesthesiologist prepared 20 mg of nefopam in 100 mL of normal saline or 100 mL of normal saline alone for the nefopam or the control group, respectively. Both solutions were clear; thus, the other anesthesiologist who took care of patients in the operating room could not distinguish them.
All patients received 0.05 mg/kg of midazolam intravenously to relieve preoperative anxiety. When they arrived at the operating room, routine monitoring with electrocardiogram, pulse oximetry, and noninvasive arterial pressure was initiated. Alfentanil, propofol, and rocuronium were used for the induction of general anesthesia. While the patient was breathing with 100% oxygen, 5 μg/kg of alfentanil and 2 mg/kg of propofol were injected. After confirming loss of patient consciousness, 0.6 mg/kg of rocuronium was given. Patient lungs were ventilated manually with 6 vol% of sevoflurane in 100% oxygen for a few minutes. A supraglottic airway was inserted to secure the airway, and mechanical ventilation was initiated. During skin preparation and surgical draping, each patient was given the prepared drug, which was administered intravenously over 15 min.
Additional 5 μg/kg of alfentanil was given immediately before starting the operation, and sevoflurane in an oxygen and air mixture (inspired fraction of oxygen = 0.5) was controlled to maintain arterial pressure and heart rate within 80% to 120% of baseline values during the operation. At the end of the main procedure, 30 mg of intravenous ketorolac was given to all the patients preventively. At the end of surgery, after confirming recovery of muscle power and consciousness, the supraglottic airway was removed, and the patient was transferred to the postanesthetic care unit (PACU).
We asked patients to rate their pain intensity on an 11-point numerical rating scale (NRS), from 0 to 10. When patients reported NRS of 5 or higher, 0.5 μg/kg of fentanyl was administered at the PACU. In the general ward, 30 mg of ketorolac was given intravenously to patients whose NRS for pain was 5 or more up to twice a day and with a 4-h window. An oral analgesic drug, 7.5 mg of meloxicam, was prescribed to all patients as soon as they started eating at postoperative 6 h. Patients took meloxicam daily during the next 5 days.
The primary outcomes were the NRS for pain and the administration of rescue analgesic drugs. The NRS was recorded at the PACU, and postoperative 6 and 24 h during the hospitalization period. The number of times each patient received the rescue analgesic drug in each time frame was also recorded. On postoperative 10 days and at 3 months, the NRS was evaluated in an outpatient clinic. As secondary outcomes, we investigated the following factors: the implementation of postoperative adjuvant chemotherapy, radiotherapy (RT), or hormone therapy, and any complication following the breast surgery, such as lymphedema, infection, seroma, hematoma, and axillary web syndrome.
Statistical Analysis
The proportion of patients who received fentanyl at the PACU due to NRS of 5 or higher was regarded as the main effect variable used for power analysis. In our pilot survey, the proportion was ∼60%, and 42 patients in each group would be sufficient to detect a decrease of 30% (α = 0.05, β = 80%). Assuming an overall rate of attrition of 10%, we calculated that 47 patients per group were needed.
We performed the Shapiro–Wilk test to determine the distribution of each variable (results not shown). Student t test or the Mann–Whitney U test, repeated-measures ANOVA, and the χ2 test were performed as appropriate. We used the SPSS software for analysis (ver. 21; IBM Co., Armonk, NY), and P values < 0.05 were considered to indicate statistical significance.
RESULTS
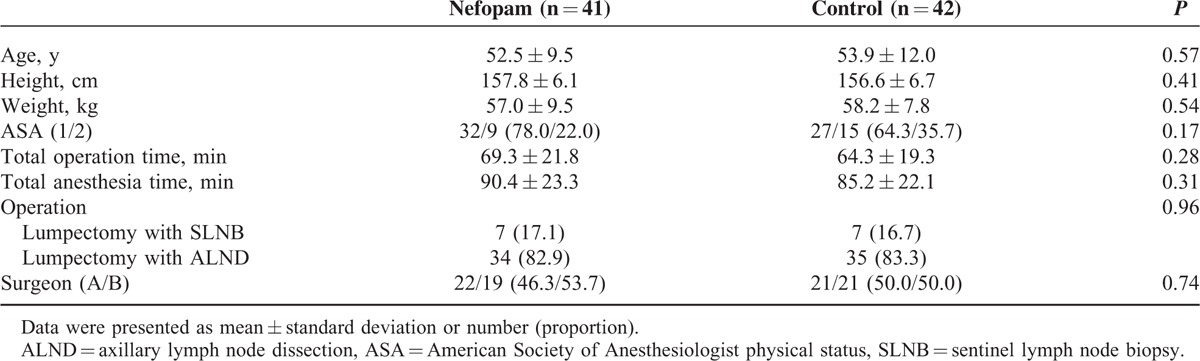
In total, 94 patients were enrolled, and each was initially assigned to the control group or the nefopam group according to the predetermined random order. Of these, 11 patients dropped out of the study, and the control and the nefopam groups finally included 42 and 41 patients, respectively (Figure 1). Basal characteristics of patients, surgery, and anesthesia were comparable between the 2 groups (Table 1).
FIGURE 1.
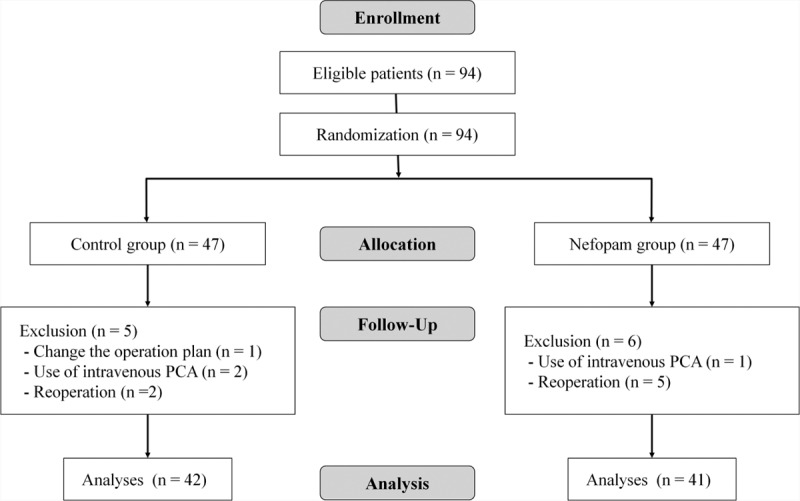
Flow diagram of patients’ enrollment. PCA = patient-controlled analgesia.
TABLE 1.
Characteristics of Patient, Surgery, and Anesthesia
Postoperative Pain and Consumption of Rescue Analgesic Drugs
The NRS for postoperative pain was significantly lower in the nefopam group than in the control group in the PACU (4.5 ± 2.2 vs 5.7 ± 1.5, respectively; P = 0.005), at postoperative 6 h (3.0 ± 1.6 vs 4.5 ± 1.3, respectively; P < 0.001), and at postoperative 24 h (3.1 ± 1.1 vs 3.8 ± 1.5, respectively; P = 0.01). However, it was comparable between the 2 groups at postoperative 10 days (1.0 ± 1.2 vs 1.2 ± 1.6, respectively; P = 0.55) and at postoperative 3 months (0.6 ± 1.0 in the nefopam group vs 0.8 ± 1.0 in the control group, respectively; P = 0.31; Figure 2).
FIGURE 2.
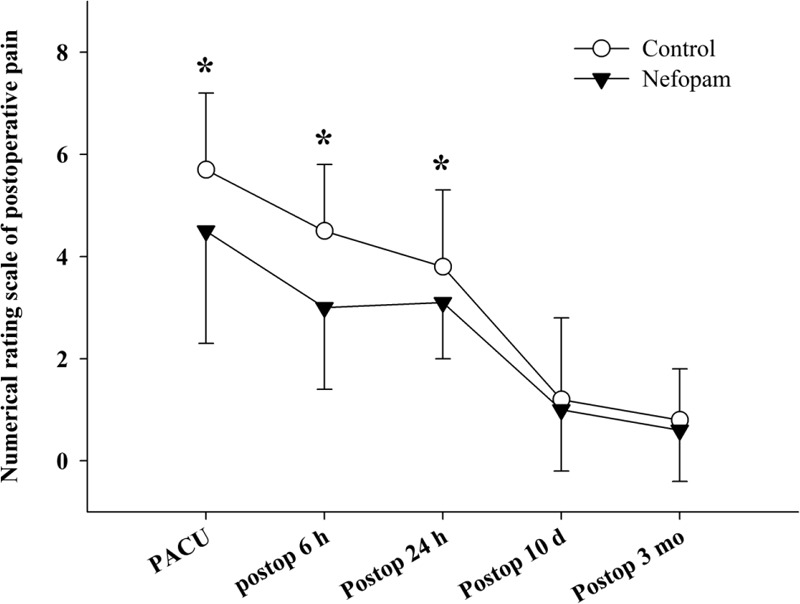
Numerical rating scale of postoperative pain. PACU = postanesthetic care unit, postop = postoperative. ∗P < 0.05.
When we sorted patients who reported any postoperative discomfort regardless of the NRS value for postoperative pain, the proportion was not significantly different between groups at postoperative 10 days (53.7% in the nefopam group vs 61.9% in the control group, P = 0.45). However, significantly fewer patients suffered from postoperative pain in the nefopam group than in the control group at postoperative 3 months (36.6% vs 59.5%, P = 0.04) (Table 2), even though the NRS for pain was low and comparable between the 2 groups at this time.
TABLE 2.
Number of Patients Who Complained of Postoperative Pain
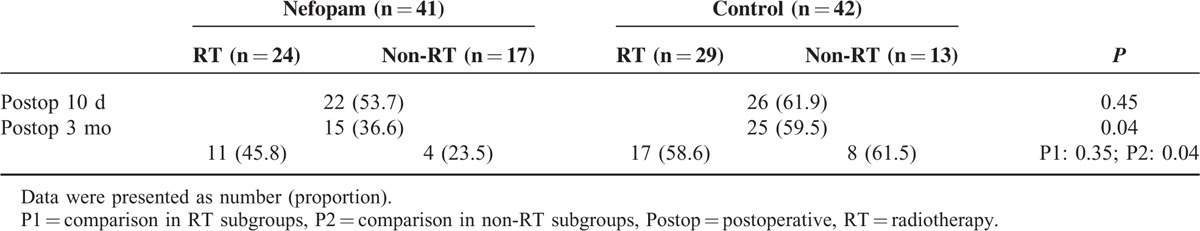
Patients in each group were subdivided into the RT or non-RT group by whether they had undergone postoperative radiation therapy at postoperative 3 months. In the cohort with postoperative adjuvant RT, the proportion of patients presenting with chronic pain were not different between the 2 treatment groups (45.8% in the nefopam group vs 58.6% in the control group, P = 0.35); however, in the non-RT subgroups, significantly fewer patients experienced chronic pain in the nefopam group than in the control group (23.5% in the nefopam group vs 61.5% in the control group, P = 0.04; Table 2).
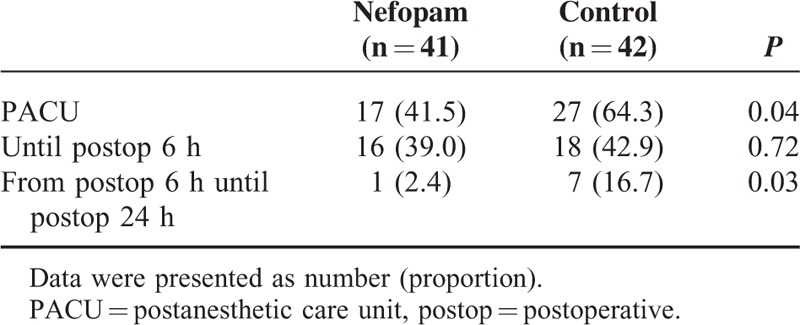
Significantly fewer patients in the nefopam group received fentanyl in the PACU compared with the control group (41.5% vs 64.3%, respectively, P = 0.04). Comparable numbers of patients in both groups were given ketorolac until postoperative 6 h after being discharged from the PACU (39.0% in the nefopam group vs 42.9% in the control group, P = 0.72). However, the number of patients, who received ketorolac from postoperative 6 h until postoperative 24 h, was significantly lower in the nefopam group than in the control group (2.4% vs 16.7%, respectively, P = 0.03; Table 3).
TABLE 3.
Administration of Rescue Analgesic Drugs at Postoperative Period
Postoperative Adjuvant Therapy
There was no significant difference between the 2 groups in the number of patients who underwent postoperative chemotherapy (39.0% in the nefopam group vs 31.0% in the control group, P = 0.44), radiation therapy (58.5% in the nefopam group vs 69.0% in the control group, P = 0.32), or hormone treatment (31.7% in the nefopam group vs 40.5% in the control group, P = 0.41).
Postoperative Complications After Breast Cancer Surgery
During the first 3 months postoperatively, some patients presented postoperative complications as follows: seroma at the operation site (8 in the nefopam group, 6 in the control group), lymphedema of the ipsilateral arm (1 in the nefopam group); surgical site infection (1 in the control group), axillary web syndrome (1 in the nefopam group), and intercostobrachial neuralgia (1 in the control group). The overall incidence of surgical complications was not significantly different between the groups (24.4% in the nefopam group vs 19.0% in the control group, P = 0.56).
DISCUSSION
In this study, we found that acute postoperative pain after breast cancer surgery was relieved by preventive nefopam analgesia. Additionally, nefopam reduced the persistence of chronic pain at postoperative 3 months, a difference that was more definite in patients who had not undergone postoperative RT.
This is the first study to verify the analgesic efficacy of nefopam for acute and chronic pain after breast cancer surgery, and it provides a basis for integrating preventive nefopam as part of procedure-specific pain control, especially in lumpectomy with ALND or SLNB.
The analgesic efficacy of nefopam during the acute postoperative period was known from several preclinical and clinical studies.5–7 Our results were also similar to previous studies examining acute postoperative pain, which showed that patients treated with nefopam reported severe pain less often. All patients received ketorolac routinely at the end of the surgery and meloxicam in the acute postoperative period. Delage et al8 reported that nefopam and ketoprofen showed synergistic effects when they were given together. In our study, nefopam was expected to enhance the action of NSAIDs, intravenous ketorolac, and oral meloxicam. Unexpectedly, the proportion of patients who were given rescue analgesics was comparable during the first postoperative 6 h regardless of nefopam treatment. However, fentanyl use in the PACU was reduced significantly with nefopam, and the effects of fentanyl seemed to last to a certain extent.
Although several studies have reported better acute postoperative pain control by nefopam,6,7 whether preventive nefopam can affect the chronicity of postsurgical pain has rarely been studied. According to description from our patients, chronic bothersome discomfort or pain came in different forms, such as tenderness, throbbing, stinging, burning, or piercing pain, intermittently or constantly. Patients showed relatively low NRS values for these symptoms at postoperative 3 months independent of the administration of nefopam. This could result from a less complex operation, that is, lumpectomy with ALND or SLNB. More complex operations are known to contribute to chronic postoperative pain in breast cancer surgery.1 However, it is noteworthy that the proportion of patients who presented with any chronic symptom was still low in the nefopam group, regardless of the NRS level. This long-term efficacy of nefopam was maximized in patients without postoperative adjuvant RT; it showed no measurable benefit for patients with adjuvant RT. Postsurgical adjuvant RT on the breast itself can cause acute skin toxicity and breast pain,16,17 which seemed to deprive the RT group patients of the long-term effects of nefopam.
We can raise the question of how a single treatment of nefopam could affect both acute and chronic postoperative pain. First, 1 of the characteristics of preventive analgesia is that the analgesic action can last longer than expected.18 Although the preventive analgesic method differed from ours, it has been reported that a preoperative single thoracic paravertebral block could reduce chronic postmastectomy pain as well as acute postoperative pain.19,20 Blumenthal et al also found that postoperative local analgesia at the surgical site could relieve pain and the analgesic effect continued until postoperative 3 months.21 Second, Fecho et al1 found that a high dose of opioid in the PACU could result in greater pain at postopeative 1 month. Our patients treated with nefopam received less opioid in the PACU, which may have contributed to the lower occurrence of chronic pain.
Although the efficacy of preventive analgesia is inconclusive, some information is available supporting our results. NMDA receptor antagonists have been shown to be favorable for preventive analgesia.22 We still do not fully understand the analgesic mechanism of nefopam; however, nefopam has been reported to block NMDA receptor-mediated excitation.23 Moreover, triple neurotransmitter (serotonin, dopamine, and norepinephrine) reuptake inhibition has been proposed as 1 of the analgesic mechanisms of nefopam,24 which offers the possibility that nefopam could be used to treat other neuropathic pain.25 Persistent neuropathic pain can develop after breast cancer surgery26; thus, nefopam could show efficacy in reducing persistent pain.
Our patients showed quite a low rate of surgical complications, such as lymphedema or axillary web syndrome, which are known to be common after breast cancer surgery and to be related with postoperative pain.27,28 For lymphedema, the incidence has been reported to be 4% to 47%, depending in part on whether the surgery included SLNB or ALND.29–31 Only 1 patient in our study suffered from lymphedema, comprising only 1.2% of the whole enrolled group. We attributed this low incidence to the short follow-up time, as the average onset time of lymphedema is 7 months after surgery.32 According to Lacomba et al,33 axillary web syndrome developed within 2 weeks postoperatively, and it resolved by postoperative 3 months. However, Koehler et al34 reported that axillary web syndrome continued in ∼27% of patients until postoperative 12 weeks. Given these very different time frame in previous studies, it is possible that we might have missed axillary web syndrome.
This study has several limitations. First, we included only patients undergoing lumpectomy with ALND or SLNB. This sort of breast-conserving surgery may cause less pain or fewer complications than a mastectomy would. The NRS for pain was quite low at 10 days after surgery, as expected. However, we could find that the proportion of patients reporting any chronic pain was reduced by nefopam. Further studies will be required to assess our current outcome in patients undergoing mastectomy. Second, our findings showed a lower incidence of chronic pain in the non-RT subgroup with preventive nefopam analgesia. However, the populations in these subgroups were quite small. When we calculated the power with reference to our results, the power was approximately 56% at α = 0.05. A study including a larger sample size should be performed to reach a firmer conclusion for this population.
In conclusion, preventive nefopam was helpful in reducing acute postoperative pain with reduced use of rescue analgesic drugs, and it contributed to reduction in chronic pain after breast cancer surgery.
Footnotes
Abbreviations: ALND = axillary lymph nodes dissection, NMDA = N-methyl-d-aspartate, NRS = numerical rating scale, NSAIDs = nonsteroidal anti-inflammatory drugs, PACU = postanesthetic care unit, RT = radiotherapy, SLNB = sentinel lymph node biopsy.
ClinicalTrials.gov. (NCT02561468).
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Fecho K, Miller NR, Merritt SA, et al. Acute and persistent postoperative pain after breast surgery. Pain Med 2009; 10:708–715. [DOI] [PubMed] [Google Scholar]
- 2.Andersen KG, Duriaud HM, Jensen HE, et al. Predictive factors for the development of persistent pain after breast cancer surgery. Pain 2015; 156:2413–2422. [DOI] [PubMed] [Google Scholar]
- 3.Katz J, Jackson M, Kavanagh BP, et al. Acute pain after thoracic surgery predicts long-term post-thoracotomy pain. Clin J Pain 1996; 12:50–55. [DOI] [PubMed] [Google Scholar]
- 4.Kelly DJ, Ahmad M, Brull SJ. Preemptive analgesia I: physiological pathways and pharmacological modalities. Can J Anaesth 2001; 48:1000–1010. [DOI] [PubMed] [Google Scholar]
- 5.Girard P, Chauvin M, Verleye M. Nefopam analgesia and its role in multimodal analgesia: a review of preclinical and clinical studies. Clin Exp Pharmacol Physiol 2016; 43:3–12. [DOI] [PubMed] [Google Scholar]
- 6.McLintock TT, Kenny GN, Howie JC, et al. Assessment of the analgesic efficacy of nefopam hydrochloride after upper abdominal surgery: a study using patient controlled analgesia. Br J Surg 1988; 75:779–781. [DOI] [PubMed] [Google Scholar]
- 7.Du Manoir B, Aubrun F, Langlois M, et al. Randomized prospective study of the analgesic effect of nefopam after orthopaedic surgery. Br J Anaesth 2003; 91:836–841. [DOI] [PubMed] [Google Scholar]
- 8.Delage N, Maaliki H, Beloeil H, et al. Median effective dose (ED50) of nefopam and ketoprofen in postoperative patients: a study of interaction using sequential analysis and isobolographic analysis. Anesthesiology 2005; 102:1211–1216. [DOI] [PubMed] [Google Scholar]
- 9.Laboureyras E, Chateauraynaud J, Richebe P, et al. Long-term pain vulnerability after surgery in rats: prevention by nefopam, an analgesic with antihyperalgesic properties. Anesth Analg 2009; 109:623–631. [DOI] [PubMed] [Google Scholar]
- 10.Nam JS, Cheong YS, Karm MH, et al. Effects of nefopam on streptozotocin-induced diabetic neuropathic pain in rats. Korean J Pain 2014; 27:326–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aveline C, Roux AL, Hetet HL, et al. Pain and recovery after total knee arthroplasty: a 12-month follow-up after a prospective randomized study evaluating nefopam and ketamine for early rehabilitation. Clin J Pain 2014; 30:749–754. [DOI] [PubMed] [Google Scholar]
- 12.Fuller RW, Snoddy HD. Evaluation of nefopam as a monoamine uptake inhibitor in vivo in mice. Neuropharmacology 1993; 32:995–999. [DOI] [PubMed] [Google Scholar]
- 13.Verleye M, Andre N, Heulard I, et al. Nefopam blocks voltage-sensitive sodium channels and modulates glutamatergic transmission in rodents. Brain Res 2004; 1013:249–255. [DOI] [PubMed] [Google Scholar]
- 14.Marks DM, Shah MJ, Patkar AA, et al. Serotonin-norepinephrine reuptake inhibitors for pain control: premise and promise. Curr Neuropharmacol 2009; 7:331–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petrenko AB, Yamakura T, Baba H, et al. The role of N-methyl-d-aspartate (NMDA) receptors in pain: a review. Anesth Analg 2003; 97:1108–1116. [DOI] [PubMed] [Google Scholar]
- 16.Hopwood P, Haviland JS, Sumo G, et al. Comparison of patient-reported breast, arm, and shoulder symptoms and body image after radiotherapy for early breast cancer: 5-year follow-up in the randomised Standardisation of Breast Radiotherapy (START) trials. Lancet Oncol 2010; 11:231–240. [DOI] [PubMed] [Google Scholar]
- 17.Whelan TJ, Levine M, Julian J, et al. The effects of radiation therapy on quality of life of women with breast carcinoma: results of a randomized trial. Ontario Clinical Oncology Group. Cancer 2000; 88:2260–2266. [PubMed] [Google Scholar]
- 18.Rosero EB, Joshi GP. Preemptive, preventive, multimodal analgesia: what do they really mean? Plast Reconstr Surg 2014; 134 suppl 2:85S–93S. [DOI] [PubMed] [Google Scholar]
- 19.Karmakar MK, Samy W, Li JW, et al. Thoracic paravertebral block and its effects on chronic pain and health-related quality of life after modified radical mastectomy. Reg Anesth Pain Med 2014; 39:289–298. [DOI] [PubMed] [Google Scholar]
- 20.Chiu M, Bryson GL, Lui A, et al. Reducing persistent postoperative pain and disability 1 year after breast cancer surgery: a randomized, controlled trial comparing thoracic paravertebral block to local anesthetic infiltration. Ann Surg Oncol 2014; 21:795–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blumenthal S, Dullenkopf A, Rentsch K, et al. Continuous infusion of ropivacaine for pain relief after iliac crest bone grafting for shoulder surgery. Anesthesiology 2005; 102:392–397. [DOI] [PubMed] [Google Scholar]
- 22.McCartney CJ, Sinha A, Katz J. A qualitative systematic review of the role of N-methyl-d-aspartate receptor antagonists in preventive analgesia. Anesth Analg 2004; 98:1385–1400. [DOI] [PubMed] [Google Scholar]
- 23.Biella GE, Groppetti A, Novelli A, et al. Neuronal sensitization and its behavioral correlates in a rat model of neuropathy are prevented by a cyclic analog of orphenadrine. J Neurotrauma 2003; 20:593–601. [DOI] [PubMed] [Google Scholar]
- 24.Gregori-Puigjane E, Setola V, Hert J, et al. Identifying mechanism-of-action targets for drugs and probes. Proc Natl Acad Sci USA 2012; 109:11178–11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim KH, Abdi S. Rediscovery of nefopam for the treatment of neuropathic pain. Korean J Pain 2014; 27:103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jung BF, Ahrendt GM, Oaklander AL, et al. Neuropathic pain following breast cancer surgery: proposed classification and research update. Pain 2003; 104:1–13. [DOI] [PubMed] [Google Scholar]
- 27.Langford DJ, Paul SM, West C, et al. Persistent arm pain is distinct from persistent breast pain following breast cancer surgery. J Pain 2014; 15:1238–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cho Y, Do J, Jung S, et al. Effects of a physical therapy program combined with manual lymphatic drainage on shoulder function, quality of life, lymphedema incidence, and pain in breast cancer patients with axillary web syndrome following axillary dissection. Support Care Cancer 2016; 24:2047–2057. [DOI] [PubMed] [Google Scholar]
- 29.Armer J, Fu MR, Wainstock JM, et al. Lymphedema following breast cancer treatment, including sentinel lymph node biopsy. Lymphology 2004; 37:73–91. [PubMed] [Google Scholar]
- 30.Petrek JA, Heelan MC. Incidence of breast carcinoma-related lymphedema. Cancer 1998; 83 (Suppl American):2776–2781. [DOI] [PubMed] [Google Scholar]
- 31.Langer I, Guller U, Berclaz G, et al. Morbidity of sentinel lymph node biopsy (SLN) alone versus SLN and completion axillary lymph node dissection after breast cancer surgery: a prospective Swiss multicenter study on 659 patients. Ann Surg 2007; 245:452–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gergich NLS, Pfalzer LA, McGarvey C, et al. Preoperative assessment enables the early diagnosis and successful treatment of lymphedema. Cancer 2008; 112:2809–2819. [DOI] [PubMed] [Google Scholar]
- 33.Lacomba MT, Del Moral OM, Zazo JLC, et al. Axillary web syndrome after axillary dissection in breast cancer: a prospective study. Breast Cancer Res Treat 2009; 117:625–630. [DOI] [PubMed] [Google Scholar]
- 34.Koehler LA, Blaes AH, Haddad TC, et al. Movement, function, pain, and postoperative edema in axillary web syndrome. Phys Ther 2015; 95:1345–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]


