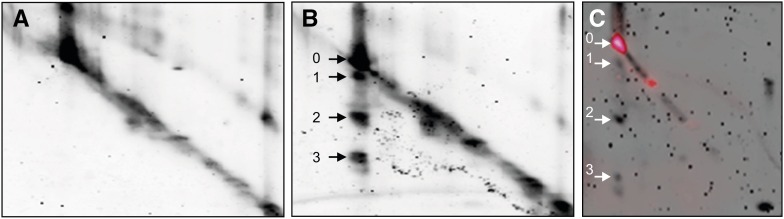
Figure 5.
2D-EMSA for the identification of substrates of Sl2- and Sl3-MMP. Apoplastic proteins were isolated from leaves of 5-week-old transgenic tomato plants silenced for Sl2/3-MMP expression and separated by SDS-PAGE. The lanes were excised, infiltrated with recombinant Sl3-MMP(His)6 in reaction buffer (B) or buffer alone (A), incubated overnight, and subjected to SDS-PAGE in the second dimension. Fluorescence (TOPLAB Ruby) staining revealed undigested proteins in the diagonal and proteins cleaved by Sl3-MMP(His)6 with increased electrophoretic mobility below (spots 1–3). Equivalent results were obtained after in-gel digestion with Sl2-MMP(His)6 (Supplemental Fig. S6). Sl2/3-MMP cleavage products (spots 1–3) and the corresponding undigested protein (spot 0) were excised and subjected to peptide fingerprint analysis by tryptic digestion and MALDI-TOF-MS (Supplemental Fig. S7). A, Buffer-infiltrated control. B, Digestion of apoplastic proteins by Sl3-MMP(His)6. C, Zymography reveals proteolytic activity for spot 0 but not for spots 1 to 3.