Abstract
Cross-sectional association has been shown between type 2 diabetes and hypothalamic–pituitary–adrenal (HPA) axis dysregulation; however, the temporality of this association is unknown. Our aim was to determine if type 2 diabetes is associated with longitudinal change in daily cortisol curve features. We hypothesized that the presence of type 2 diabetes may lead to a more blunted and abnormal HPA axis profile over time, suggestive of increased HPA axis dysregulation. This was a longitudinal cohort study, including 580 community-dwelling individuals (mean age 63.7 ± 9.1 years; 52.8 % women) with (n = 90) and without (n = 490) type 2 diabetes who attended two MultiEthnic Study of Atherosclerosis Stress ancillary study exams separated by 6 years. Outcome measures that were collected were wake-up and bedtime cortisol, cortisol awakening response (CAR), total area under the curve (AUC), and early, late, and overall decline slopes. In univariate analyses, wake-up and AUC increased over 6 years more in persons with as compared to those without type 2 diabetes (11 vs. 7 % increase for wake-up and 17 vs. 11 % for AUC). The early decline slope became flatter over time with a greater flattening observed in diabetic compared to non-diabetic individuals (23 vs. 9 % flatter); however, the change was only statistically significant for wake-up cortisol (p-value: 0.03). Over time, while CAR was reduced more, late decline and overall decline became flatter, and bedtime cortisol increased less in those with as compared to those without type 2 diabetes, none of these changes were statistically significant in adjusted models. We did not identify any statistically significant change in cortisol curve features over 6 years by type 2 diabetes status.
Keywords: Diabetes, Stress, Cortisol, HPA axis, Epidemiology
Introduction
Hypercortisolism can lead to the development of hyperlipidemia, abdominal/central obesity, and eventually to the development of type 2 diabetes mellitus [1, 2]. In patients with metabolic syndrome, higher fasting cortisol has been associated with central obesity, increased waist circumference, high triglyceride levels, hypertension, increased fasting glucose levels, and insulin resistance [3]. Patients with type 2 diabetes mellitus have evidence of subclinical hypercortisolism, as they have been found to have higher basal plasma cortisol levels [4], higher plasma cortisol levels after dexamethasone suppression test [4, 5], higher 24 h urine-free cortisol [5], and increased adrenal gland volume [6] compared to individuals without type 2 diabetes mellitus [7].
Dysregulation of the hypothalamic–pituitary–adrenal (HPA) axis has also been shown to predict the incidence of type 2 diabetes, as men with abnormal neuroendocrine secretory pattern at baseline, defined as a more blunted HPA axis profile, were more likely to develop type 2 diabetes over 5 years [8]. However, prior studies have not examined whether the presence of type 2 diabetes leads to alternations in the HPA axis profile over time. Hypercortisolism and dysregulation of the HPA axis in the setting of type 2 diabetes might have implications for glycemic control and development of diabetes complications. The prevalence of Cushing Syndrome is higher in patients with type 2 diabetes mellitus than in the general population [9, 10]. Clinical hypercortisolism in the setting of glucocorticoid administration, Cushing’s Disease, and Cushing’s Syndrome is associated with increased cardiovascular disease (CVD) risk [11, 12], vascular mortality [13], and coronary atherosclerosis [14–17]. The HPA axis is a tightly regulated system that represents one of the body’s response mechanisms to acute and chronic physiological or psychological stress. Cortisol levels typically follow a strong circadian rhythm across the day, in which levels are typically high upon waking, increase by 50–75 % during the 30–40 min post-awakening (referred to as the cortisol awakening response or CAR) [18], and decline across the remainder of the day, reaching a nadir in the late evening some 18+ h after awakening [19, 20]. Both the size of the CAR and the rate of the cortisol decline across the day represent important aspects of HPA axis functioning and have been associated with sociodemographic factors, psychosocial well-being, and physical and mental health [21–30]. The cortisol diurnal area under the curve (AUC) can be considered as measures of daily cortisol exposure [31]. In response to physiological or psychological stressors, the HPA axis is activated, resulting in the release of corticotropin-releasing hormone from the hypothalamus, which stimulates the anterior pituitary gland to release adrenocorticotropin (ACTH). ACTH stimulates release of cortisol from the adrenal glands, which results in a cascade of physiological events. Once the stressor has resolved, the response is terminated through a negative feedback loop; however, chronic physiological and psychological stressors injure this component of the stress response, resulting in subclinical hypercortisolism [32]. Hypercortislism occurs not only in patients with type 2 diabetes, but also in shift workers and patients with major depression, panic disorder, generalized anxiety disorder, alcoholism, anorexia and bulimia nervosa, obesity, polycystic ovarian syndrome, obstructive sleep apnea, and end-stage renal disease [33].
In a previous cross-sectional study of the MultiEthnic Study of Atherosclerosis (MESA) Stress I Ancillary study, we have found significant cross-sectional associations between components of the diurnal cortisol profile and type 2 diabetes status [7]. The CAR was lower in those with type 2 diabetes compared to those without type 2 diabetes, an association that was mainly seen in men [7]. The individuals with type 2 diabetes had a slower early cortisol decline slope than individuals without type 2 diabetes, an association that was, however, not significant in the fully adjusted model [7]. Women with type 2 diabetes had higher total cortisol AUC, which persisted following multivariable adjustment, while men with type 2 diabetes had a non-statistically significant lower AUC [7]. Because our study was cross-sectional we were unable to determine the temporality of these associations and whether the presence of type 2 diabetes predicted longitudinal change in HPA axis measures. However, another cross-sectional study published recently by our group from the MESA Stress II Ancillary Study suggests that type 2 diabetes may be associated with hypercortisolism and HPA axis dysfunction. We found that among individuals with type 2 diabetes, early decline slope, overall decline slope, bedtime cortisol, and AUC were significantly and positively associated with higher glycemia, assessed by HbA1c. In contrast, cortisol curve features were not associated with HbA1c among non-diabetic participants [34].
In this manuscript, based on our previously published findings, as well as other studies, we hypothesize that the presence of type 2 diabetes may lead to a more blunted HPA axis profile with a decreased CAR, a slower early cortisol decline slope and altered AUC, and bedtime cortisol over time, suggestive of increased HPA axis dysregulation. To address this question, we used longitudinal data from the MESA Stress I and II Ancillary Studies to examine the association of type 2 diabetes status with longitudinal change in daily cortisol curve features over a 6-year period.
Subjects and methods
Study population
Details of the study, sampling and cohort examination procedures have been published in details elsewhere [7, 35]. In brief, MESA is a multi-center, multiethnic longitudinal cohort study of the prevalence and correlates of subclinical cardiovascular disease and the factors that influence its progression [35]. Between July 2000 and August 2002, 6814 men and women without clinical cardiovascular disease who identified themselves as White, Black, Hispanic or Chinese, and were 45–84 years of age were recruited from six U.S. communities: Baltimore City and Baltimore County, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles County; and St. Paul, Minnesota. The first (baseline) visit was conducted in 2000–2002. Follow-up visits 2, 3, 4, and 5 were done in 2002–2004, 2004–2005, 2005–2007, and 2010–2012, respectively.
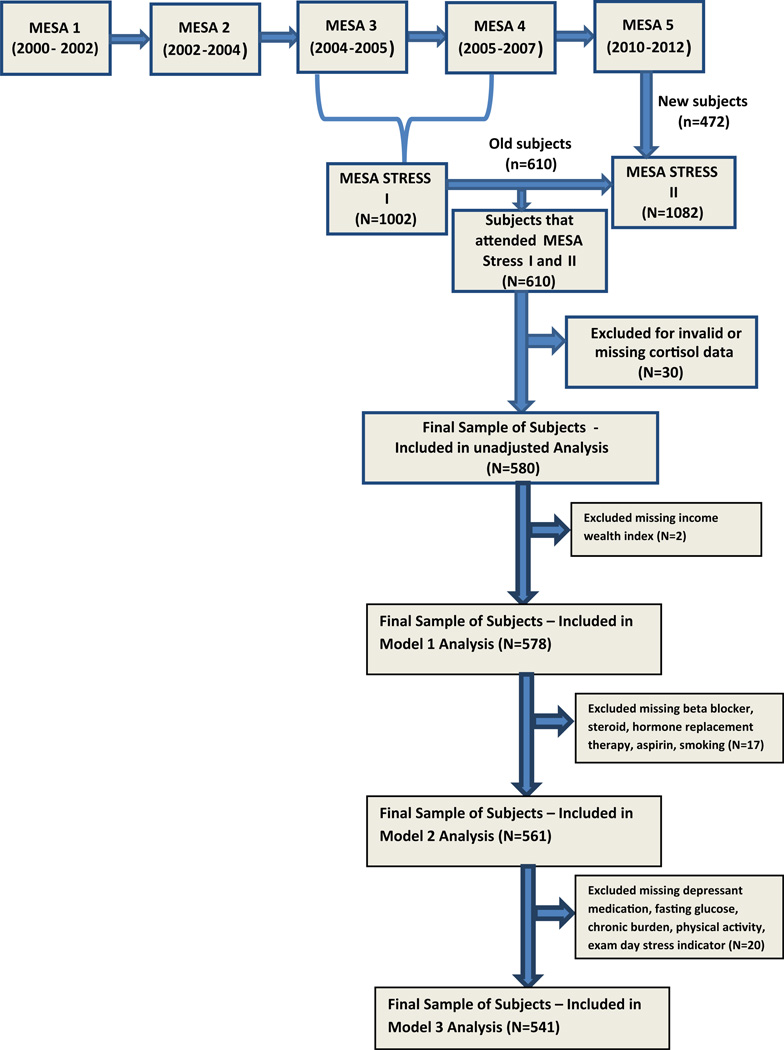
The MESA Stress Ancillary Studies (I and II) collected detailed measures of stress hormones, including salivary cortisol samples, on a subset of participants at two time periods approximately 6 years apart. From 2004 to 2006, MESA Stress I was initiated, recruiting 1002 White, Hispanic, and African-American participants from the New York and Los Angeles sites during MESA exams 3 and 4 [7]. Between 2010 and 2012, MESA Stress II was conducted, recruiting 1082 individuals from the New York, Los Angeles, and Baltimore sites during MESA exam 5. There were 610 individuals who attended both MESA Stress Exams I and II who were the subject of this analysis (Fig. 1). Invalid daily cortisol samples including samples with missing cortisol value or unreliable cortisol values (0 or >100 nmol/L) or missing time of sample collection were excluded, which lead to a further exclusion of N = 30 participants who had no valid samples on any exam day for at least one wave. The final crude analyses contained 580 adults. Night shift workers were excluded from recruitment for this study since their normal diurnal cortisol profiles would be altered by their awakening in the mid-day and/or evening. All participants provided informed consent and the above studies were approved by the Institutional Review Boards of each institution.
Fig. 1.
Multiethnic study of atherosclerosis (MESA) stress ancillary study consort diagram
Exposure: type 2 diabetes status at MESA stress I
Type 2 diabetes status was assessed and defined at MESA Stress I. All patients fasted overnight and avoided any intense physical activity or smoking for at least 2 h prior to the blood draw. Fasting glucose values were obtained between 0730 and 1030 h and serum was stored at −70 °C as previously described [35]. Serum glucose is measured by rate reflectance spectrophotometry using thin film adaptation of the glucose oxidase method on the Vitros analyzer (Johnson & Johnson Clinical Diagnostics, Inc., Rochester, NY 14650). Type 2 diabetes was defined as a single fasting glucose value ≥126 mg/dL, or use of oral hypoglycemic medication, insulin, or both [7].
Outcome: salivary cortisol curve features
In MESA Stress I, participants collected six salivary cortisol samples per day, immediately upon awakening (and before getting out of bed), 30 min after awakening, at 1000, 1200 h, or before lunch (whichever was earlier), at 1800 h or before dinner (whichever was earlier), and at bedtime. Participants collected the above samples for 3 successive weekdays. Therefore each participant provided 18 samples overall. In MESA Stress II, participants collected eight salivary cortisol samples over 2 days with 8 time points measured per day. Samples were taken immediately after waking, 30 min after wake-up, 1 h after breakfast, at 1000 h, at 1200 h or before lunch (whichever was earlier), 1600, 1800 h or before dinner (whichever was first), and at bedtime. Salivary cortisol is considered by many as the best approach to evaluate individuals for endogenous hypercortisolism [36]. Moreover, salivary cortisol has been found to be superior to plasma cortisol in the evaluation of adrenal function in individuals with type 2 diabetes as it is not influenced by overall glycemic control (HbA1c) or daily glucose variability [37]. From our work in MESA Stress I, we learned that we could adequately characterize the diurnal cortisol curve with 2 days of sample collection, thus we eliminated the third day to reduce participant burden. In MESA Stress II, sample collection times corresponded to those in MESA Stress I, with the following exception—two additional samples were collected at 1 h after breakfast and 1800 h to better characterize the early and late decline slopes, respectively [30]. Participants recorded wake-up time by answering a questionnaire. As previously reported in MESA, 97 % of participants collected samples on all 3 days and 85 % of participants collected at least 5 samples per day for all days on which they collected samples [28]. Based on prior work in our population, the median difference in times between the track caps and recorded times was between 2 and 4 min depending on the sample. The 25th and 75th percentiles were between 1 and 2 and 5 and 13 min, respectively, with the longest times corresponding to the last sample of the day. Therefore the accuracy of the CAR was most affected by timing discrepancy. Overall the first sample was taken within 5 min of wake-up for 78 % of days across participants and the median difference between the first and second sample was 34 min [31, 38]. We are therefore confident about adherence to the sampling protocol. While lower compliance with the collection protocol was associated with a less pronounced CAR, compliance was not associated with any other cortisol features and adjustment for compliance did not affect the associations of cortisol features with sociodemographic characteristics (17).
Saliva cortisol samples were collected using cotton swabs. Participants were permitted to carry samples with them over the course of the day during collection but were instructed to store the samples in the refrigerator after collection until they were returned to the clinic in-person or via mail (within 1 week of collection). Prior work has shown that salivary cortisol samples are stable at room temperature for at least 1 week and can be returned via mail [39]. Samples were stored at −20 °C until they were analyzed. Prior to the analysis, samples were thawed and centrifuged at 3000 rpm for 3 min in order to obtain saliva with low viscosity. Cortisol samples were analyzed using a chemiluminescence assay with a high sensitivity of 0.16 ng/mL (IBL-Hamburg; Germany). Intra- and interassay coefficients of variation are below 8 % [7].
We investigated seven features of the daily cortisol curve: wake-up and bedtime cortisol levels, CAR, standardized total AUC, early decline slope, late decline slope, and overall decline slope. The CAR was calculated as the difference between the wake-up cortisol levels and the levels at 30 m in post-awakening. The early decline slope (between 30 min and 2 h post-awakening) and late decline slope (between 2 h post-awakening and bedtime) were calculated as the average hourly rate of decline for the given time period. The overall decline slope was calculated as the rate of decline from the wake-up time to the bedtime (excluding the 2nd sample). To calculate the AUC, we used linear splines to connect the values from each of the sample times and then calculated the area under the linear spline based on the trapezoid rule [40], using all available data and restricting estimates to a 16-h day duration for all participants. The AUC was then standardized by the length of duration (which is 16 h in our analysis). Each of the features was computed on a daily basis; 99 % (N = 573), 92 % (N = 535), 97 % (N = 561), 99 % (N = 576), and 97 % (N = 562) of participants had sufficient data from at least 1 day at each wave of studies (3 days for MESA Stress I and 2 days for MESA Stress II) to calculate wake-up cortisol, CAR, early decline slope, late decline slope, and AUC measures, respectively.
Covariates
We adjusted for variables considered as potential confounders in the diabetes–cortisol association from the baseline MESA Stress I Exam. Covariates such as age, sex, race/ethnicity, cigarette smoking, highest levels of education, and annual income were self-reported, using protocols as previously published [7, 35]. Indicators of socioeconomic status were combined into a single wealth income index [28]. Participants were categorized as current or not current smokers, as it has been shown that smokers had higher cortisol levels than not current smokers, where there are no differences in cortisol values among ex-smokers or never smokers [41]. Over the counter and prescription medication history was collected in each office visit [7, 35]. Waist circumference was measured at the minimum abdominal girth [7]. Physical activity was assessed using the 28-item MESA Typical Week Physical Activity Survey (TWPAS) [42]. We summarized physical activity as the MET-min/week spent in moderate to vigorous exercise. Depressive symptoms were assessed using the Center for Epidemiological Studies Depression (CES-D) scale as previously described [43]. We assessed stress perception on the day of sample collections based on the following question and choices: “How typical was this day for you re: stress/pressure?” with the following choices “1: Today was typical in terms of my workload and stress level; 2: Today I had a greater workload, felt more stressed than usual; 3: Today I had a lower workload, felt less stressed than usual.” Chronic burden was assessed using the Chronic Burden Scale [44]. Respondents were asked to indicate whether they had experienced ongoing problems for ≥6 months in five domains and to rate if it was moderately or very stressful—health (self), health (loved one), job, relationship, and finances. We summed the number of domains in which chronic burden was experienced (0, 1, 2, or more) to estimate overall chronic burden [45].
Statistical analysis
Longitudinal models were used to formally investigate the crude and adjusted association between type 2 diabetes status and the change in the cortisol daily curve. In these models, type 2 diabetes status at baseline (MESA Stress I) was the primary exposure of interest, and daily salivary cortisol levels at two different exams were the outcome measures. Due to its skewed distribution, cortisol was log-transformed before the cortisol features were calculated [28, 46, 47]. Because multiple salivary cortisol samples were taken within the same day at each exam, time of day when the cortisol sample was collected was modeled using a piecewise linear spline. The piecewise linear splines had two knots at the inflection points of the daily curve (30 min and 2 h after wake-up [28], resulting in a 3-piece spline model in that captured the non-linearity of the cortisol daily profile. Compared with modeling change in each cortisol feature separately, this approach allowed us to estimate changes in all curve features simultaneously using all the available daily salivary cortisol samples from all days at both exams (i.e., up to 34 data points per subject in MESA Stress I [6 samples/day × 3 days] and MESA Stress II [8 samples/day × 2 days), and is more statistically efficient [30]. The difference in the annual change of each cortisol feature was then derived from the relevant model coefficients (see Supplementary Table 1). We used random effects to model the correlations among samples within individuals. Robust standard errors are reported. In addition, a likelihood ratio test was used to make a global judgment on the difference in the change of the entire cortisol daily curve over time between those with and without type 2 diabetes. The model specifications are included in Appendix.
In the unadjusted model, we examined the association of type 2 diabetes status with change in cortisol daily curve features over 6 years without adjusting for any other factors. Model 1 was adjusted for sociodemographic factors (age, sex, race/ethnicity, and socioeconomic status); Model 2 was further adjusted for waist circumference, depressive symptoms (continuous CES-D score), smoking status, and medication usage including usage of beta-blocker, aspirins, inhaled or oral steroids, and hormone replacement therapy. We also examined whether the association of type 2 diabetes mellitus with change in cortisol curve features was modified by sex or race/ethnicity. Because there was no evidence of interaction by sex or race/ethnicity, we present the results for the overall cohort. Finally global tests were further conducted to test whether the change in the entire cortisol daily curve over time differed in diabetic compared to non-diabetic individuals. The global test is a likelihood ratio test that compared the goodness of fit (in terms of log-likelihood) of the full model that includes type 2 diabetes, the splines, and the interactions of type 2 diabetes with the splines, and time between visits to a nested model that includes everything in the full model except excluding the three-way interaction terms between type 2 diabetes, the splines, and time between visits and the interaction term between type 2 diabetes and time between visits. A p-value is calculated by comparing the value of the log-likelihood ratio statistics for the given dataset that is used to fit the models to a critical value from a Chi-square distribution (with its degree of freedom equal to the difference in the number of terms fitted in the full and the nested model which is 4 in our case) to decide whether to reject the nested model in favor of the full model (i.e., if the p-value of the likelihood ratio test is <0.05, it means that it is more likely that the full model is a better fit for the data than the nested model) [48]. Statistical significance was defined as a 2-sided alpha <0.05. Analyses were conducted using Statistical Analysis Software (SAS), version 9.2.
Results
Population characteristics
The final analyses contained 580 adults (mean age: 63.7 ± 9.1, range 48–87 years) and provided 2883 days of cortisol data over the two waves, representing an ethnically and socio-economically diverse group of participants—African-American (27.6 %), Hispanic-American (54.1 %), and Non-Hispanic White (18.3 %) with an approximately even distribution of women (52.8 %) and men (47.2 %). Among the 580 individuals, 18 % (n = 90) had type 2 diabetes and the mean body mass index was 29.1 ± 5.3 kg/m2. Of our participants with type 2 diabetes, 60 were taking oral hypoglycemic agents and 11 were taking insulin. Compared to individuals without type 2 diabetes, those with type 2 diabetes were slightly older, less likely to be White, had a lower income wealth index, were not likely to be using beta-blockers and aspirin, and had a higher waist circumference and fasting glucose (Table 1).
Table 1.
Baseline characteristics at MESA Stress I for individuals with and without type 2 diabetes
| Diabetes (n = 90) | Non-diabetes (n = 490) | p-value† | |||
|---|---|---|---|---|---|
| Age (mean, SD) | 65.9 (8.51) | 63.3 (9.21) | 0.01 | ||
| Sex (N, %) | 0.73 | ||||
| Female | 46 | 51 % | 260 | 53 % | |
| Male | 44 | 49 % | 230 | 47 % | |
| Race/ethnicity (N, %) | <0.01 | ||||
| Non-Hispanic Whites | 4 | 4 % | 102 | 21 % | |
| African-Americans | 33 | 37 % | 127 | 26 % | |
| Hispanic Americans | 53 | 59 % | 261 | 53 % | |
| Smoking status‡ (N, %) | 0.26 | ||||
| Never | 38 | 42 % | 238 | 49 % | |
| Former | 46 | 51 % | 205 | 42 % | |
| Current | 6 | 7 % | 45 | 9 % | |
| Income wealth index‡ (N, %) | <0.01 | ||||
| (0–1) | 23 | 26 % | 76 | 16 % | |
| (2–3) | 32 | 36 % | 124 | 25 % | |
| (4–6) | 20 | 22 % | 212 | 43 % | |
| (7–8) | 15 | 17 % | 76 | 16 % | |
| Beta-blocker‡ (N, %) | 27 | 30 % | 63 | 13 % | <0.01 |
| Steroid use (inhale/oral)‡ (N, %) | 5 | 6 % | 14 | 3 % | 0.20 |
| Hormone replacement therapy (estrogen, progestins, premarin)‡ (N, %) | 3 | 3 % | 21 | 4 % | 0.66 |
| Aspirin‡ (N, %) | 43 | 48 % | 146 | 30 % | <0.01 |
| Center for Epidemiological Studies Depression Score (median, interquartile range) | 6 | (11) | 6 | (10) | 0.18 |
| Waist circumference (cm) (mean, SD) | 107 (14.1) | 98.7 (13.8) | <0.01 | ||
| Fasting glucose (mg/dL) (mean, SD) | 141 (48.1) | 93.1 (9.97) | <0.01 | ||
| Follow-up years (mean, SD) | 5.96 (0.66) | 6.15 (0.71) | 0.01 | ||
p-value <0.05 indicates a statistically significant difference in the distribution of baseline individual characteristics between diabetes and non-diabetes groups using two-sample t test for continuous variables and Chi-square test for categorical variables
Missing values in income wealth index (N = 2), beta-blocker (N = 14), steroid use (N = 14), hormone replacement therapy (N = 14), aspirin (N = 1), smoking (N = 2)
Change in cortisol curve features by type 2 diabetes status
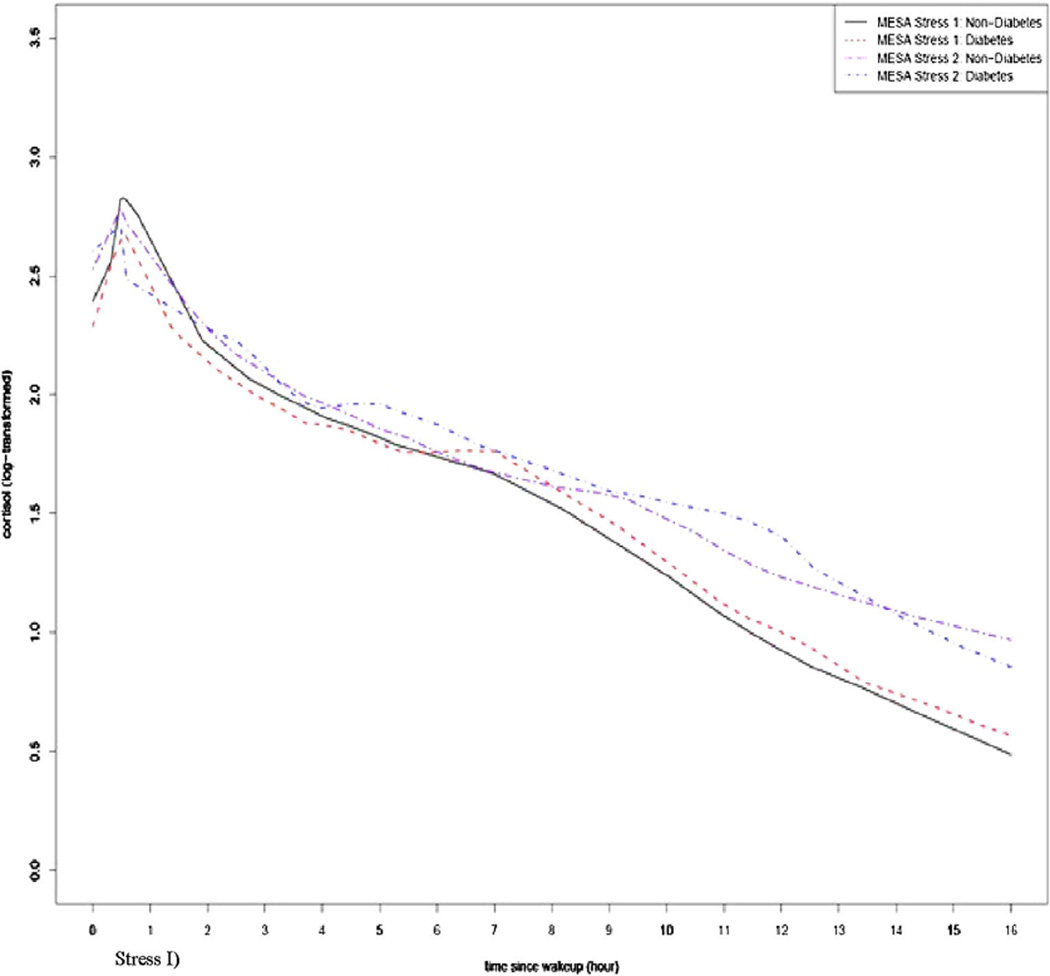
The smoothed locally estimated scatter plot smoothing (LOESS) curves of the cortisol daily profiles at the MESA Stress I and II visits stratified by type 2 diabetes status are shown in Fig. 2. In general, the direction of change in daily cortisol curves over time for those with and without type 2 diabetes were similar where both groups showed increased wake-up cortisol, lower CAR, flattened early and late decline slope, and increased AUC at the second assessment compared to the first; however, there was a difference in the degree of change over time for those with as compared to those without type 2 diabetes. Univariate analyses for the selected features of the daily cortisol curve at both waves of the MESA Stress Study and the percent change over time in features for those with and without type 2 diabetes at MESA Stress I are shown in Table 2. The mean wake-up time was 06.41 (standard deviation 1.17 h). In unadjusted analysis, wake-up values increased more in diabetic as compared to non-diabetic individuals (11 vs. 7 % respectively, p = 0.03) (Table 2). Although not statistically significant, the CAR was lower in individuals with as compared to those without type 2 diabetes (−79 vs. −47 %; p = 0.29). The early decline slope was less pronounced in both groups (as indicated by a less negative slope) and even though the flattening was greater in diabetic individuals (23 vs. 9 % increase in the slope, indicating a less negative, i.e., flatter, slope) these changes were not statistically significant (p = 0.93). Both the late decline and the overall decline slopes became flatter over time in both groups, a change that was not statistically significant between those with and without type 2 diabetes (16 vs. 31 % for the early decline slope; p = 0.93 and 9 vs. 17 % for the overall slope, p = 0.40). AUC and bedtime cortisol increased in both groups but there was not a statistically significant difference between those with and without type 2 diabetes (p = 0.48 and p = 0.86, respectively; Table 2).
Fig. 2.
Daily cortisol profile at MESA I and II by participants’ diabetes status at baseline (MESA Stress I)
Table 2.
Summary of selected cortisol features for both waves of the study and the change over time for the entire population and for each diabetes status group
| MESA stress visit |
Wake-up | CAR | Early decline slope |
Late decline slope |
Overall decline slope |
AUC 16 h | Bedtime | |
|---|---|---|---|---|---|---|---|---|
| Diabetes status | ||||||||
| No (n = 490) | I | 2.39 | 0.43 | −0.46 | −0.13 | −0.12 | 1.50 | 0.58 |
| II | 2.56 | 0.23 | −0.42 | −0.09 | −0.10 | 1.67 | 1.05 | |
| % change | 7 | −47 | 9 | 31 | 17 | 11 | 81 | |
| Yes (n = 90) | I | 2.36 | 0.34 | −0.32 | −0.11 | −0.11 | 1.53 | 0.72 |
| II | 2.62 | 0.07 | −0.25 | −0.09 | −0.10 | 1.79 | 1.16 | |
| % change | 11 | −79 | 23 | 16 | 9 | 17 | 61 | |
| p-value | 0.03 | 0.29 | 0.93 | 0.40 | 0.18 | 0.48 | 0.86 |
Trimmed mean (by excluding the top and bottom 1 % extreme values) is calculated for each of the selected cortisol features
Due to its skewed distribution cortisol being log-transformed before the cortisol features were calculated for each participant on each exam day [16, 21, 22]
p-values were calculated based on individual’s cortisol feature change since baseline using 2-sample t test between the diabetes and non-diabetes groups. This test is referred to as the unadjusted tests in an exploratory analysis
Change in cortisol features (per year) in those with as compared to those without type 2 diabetes status, adjusting for demographic, health-related, and stress factors is shown in Table 3. Overall, however, there were no statistically significant differences in the change of daily cortisol curve features in those with as compared to those without type 2 diabetes, except for a weak signal suggesting greater increases in wake-up cortisol in those with as compared to those without type 2 diabetes (p-value < 0.1; Table 3). A global test was further conducted to test whether the change in the entire cortisol daily curve over time differed in diabetic compared to non-diabetic individuals; however, the result was not significant either (Table 3). Because steroids (inhaled glucocorticoids and hormone replacement therapy) may influence cortisol curve features, we repeated our analysis excluding those on inhaled oral steroids (n = 19), estrogen (n = 23), progestin (n = 8), or missing steroid use data (n = 14). The results were nearly identical to the main analysis (Table 4). In contrast to our cross-sectional findings, we did not find effect modification by sex in these longitudinal analyses.
Table 3.
Difference in the cortisol feature change (change per year) between diabetes status groups
| Difference in the change of cortisol features by diabetes group | Unadjusted model |
Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|---|---|
| Est. | p-value | Est. | p-value | Est. | p-value | Est. | p-value | |
| Diabetes | ||||||||
| Wake-up | 0.034 | 0.083 | 0.034 | 0.083 | 0.035 | 0.075 | 0.024 | 0.214 |
| CAR | −0.029 | 0.106 | −0.029 | 0.111 | −0.028 | 0.123 | −0.020 | 0.296 |
| Bedtime | −0.001 | 0.971 | 0.000 | 0.990 | −0.002 | 0.931 | −0.005 | 0.777 |
| Early decline slope | 0.007 | 0.600 | 0.007 | 0.628 | 0.009 | 0.505 | 0.003 | 0.831 |
| Late decline slope | −0.001 | 0.437 | −0.001 | 0.471 | −0.002 | 0.252 | −0.001 | 0.465 |
| Overall decline slope | −0.002 | 0.114 | −0.002 | 0.124 | −0.002 | 0.063 | −0.001 | 0.141 |
| AUC | 0.008 | 0.595 | 0.008 | 0.583 | 0.010 | 0.490 | 0.003 | 0.842 |
| Global test on cortisol daily curvea | 0.313 | 0.420 | 0.363 | 0.670 | ||||
Model 1: Adjusted for sociodemographic factors (age, sex, race/ethnicity, and socioeconomic status)
Model 2: Adjusted for Model 1 and waist circumference, depressive symptoms, smoking status, and medication usage
Model 3: Adjusted for Model 2 and time-varying fasting glucose, anti-depressant medication use, physical activity, and typical stress day and baseline (MESA Stress I) chronic burden
The change is annual change in log-unit cortisol feature (as all cortisol features were estimated based on log-transformed cortisol values)
A likelihood ratio test was used to test whether the change in the entire cortisol daily curve over time differed in diabetic compared to non-diabetic individuals. The likelihood ratio test compared a model including diabetes, the splines, the interactions of diabetes with the splines, and time between visits to a model that added three-way interactions between diabetes, splines, and time
Table 4.
Difference in the cortisol feature change (change per year) between diabetes status groups (excluding individuals taking glucocorticoids and/or sex steroids)
| Difference in the CHANGE of cortisol features by diabetes group (exclude steroids and estrogens and progestin) |
Unadjusted modela |
Model 1a | Model 2a | Model 3a | ||||
|---|---|---|---|---|---|---|---|---|
| Est. |
p- value |
Est. |
p- value |
Est. |
p- value |
Est. |
p- value |
|
| Wake-up | 0.032 | 0.120 | 0.032 | 0.120 | 0.033 | 0.112 | 0.028 | 0.186 |
| CAR | −0.027 | 0.168 | −0.026 | 0.173 | −0.027 | 0.160 | −0.018 | 0.360 |
| Bedtime | −0.004 | 0.834 | −0.003 | 0.863 | −0.003 | 0.886 | −0.004 | 0.816 |
| Early decline slope | 0.012 | 0.366 | 0.012 | 0.385 | 0.012 | 0.368 | 0.002 | 0.874 |
| Late decline slope | −0.002 | 0.167 | −0.002 | 0.180 | −0.002 | 0.181 | −0.001 | 0.393 |
| Overall decline slope | −0.002 | 0.054 | −0.002 | 0.057 | −0.002 | 0.057 | −0.002 | 0.110 |
| AUC | 0.011 | 0.491 | 0.011 | 0.484 | 0.011 | 0.466 | 0.005 | 0.729 |
| Global test on cortisol daily curveb | 0.263 | 0.386 | 0.374 | 0.632 | ||||
The change is annual change in log-unit cortisol feature (as all cortisol features were estimated based on log-transformed cortisol values)
Model 1: Adjusted for sociodemographic factors (age, sex, race/ethnicity, and socioeconomic status)
Model 2: Adjusted for Model 1 and waist circumference, depressive symptoms, smoking status, and medication usage (no steroid or HRT adjusted due to exclusion of subject on steroids and estrogens and progestin)
Model 3: Adjusted for Model 2 and time-varying fasting glucose, anti-depressant medication use, physical activity, and typical stress day and baseline (MESA Stress I) chronic burden
N = 525 in Model 0; N = 523 (excluding N = 2 with missing income wealth index removed) for Model 1; N = 520 (excluding N = 1 with missing aspirin use and N = 2 with missing smoking status) for Model 2; N = 503 for Model 3 (excluding missing values in N = 1 depressant medication, N = 1 fasting glucose (missing only at MESA Stress II), N = 8 chronic burden, N = 10 physical activity, N = 7 typical stress day indicator (missing for all days in an exam), N = 1 aspirin, N = 2 smoking and N = 2 income wealth index)
A likelihood ratio test was used to test whether the change in the entire cortisol daily curve over time differed in diabetic as compared to non-diabetic individuals. The likelihood ratio test compared a model including diabetes, the splines, the interactions of diabetes with the splines, and time between visits to a model that added three-way interactions between diabetes, splines, and time
Because our findings may have been affected by individuals who were non-diabetic at MESA Stress I developing type 2 diabetes during the 6-year follow-up interval to MESA Stress II, we performed a subsidiary analysis exclude the 37 individuals who developed incident type 2 diabetes. Our findings were nearly identical in those 543 participants as in the total sample (n = 580; data not shown). Finally, we also performed several subsidiary analyses. When we included adjustment for baseline measure of the diurnal cortisol curve features in the multivariable model, our findings were nearly identical to our original results (data not shown). The results were similar to those without adjusting for the approximated wake-up time except that we observed a significant difference in the overall decline slope between individuals with and without type 2 diabetes in Model 2, which suggests the potential role of the wake-up time in shaping the daily cortisol curve. However, this significant difference in the overall decline slope disappeared in Model 3 when additional adjustments for time-varying fasting glucose, anti-depressant medication use, physical activity, and typical stress day and baseline (MESA Stress I) chronic burden were applied (Supplemental Table 2).
Discussion
To our knowledge this is the first study to examine the association of type 2 diabetes status with longitudinal changes in daily cortisol curve features. Overall there were no statistically significant differences in the change of daily cortisol curve features in those with type 2 diabetes compared to those without type 2 diabetes over a 6-year period after adjustment for sociodemographic factors, waist circumference, depressive symptoms, smoking status, and use of medications that may impact HPA axis function.
Prior studies have yielded conflicting results on the cross-sectional association between type 2 diabetes status and diurnal cortisol curve features [47, 49–52]. Similar to our findings, Vreeburg et al. [51], found no association between type 2 diabetes and diurnal cortisol slope in 491 individuals who did not have any mental illness and participated in the Netherlands Study of Depression and Anxiety. In contrast, in a study of individuals without a history of HPA axis dysregulation or mental illness, Lederbogen et al. [50] found an association between type 2 diabetes status and flatter daily cortisol levels in 979 individuals. A recent cross-sectional study reported similar findings [52]. Hackett et al. reported a flatter slope in cortisol decline across the day and higher bedtime cortisol levels even in their fully adjusted model [52]. We [47] and others [49] have reported a blunted CAR among individuals with type 2 diabetes compared to controls in cross-sectional analyses; however, in the present study we did not find an association between diabetes status and change in CAR over time following multivariable adjustment. A possible explanation for different findings in the cross-sectional compared to the longitudinal study may be due to the fact that a longer time interval is required to see more significant changes in the CAR attributable to type 2 diabetes status. In a recently published study that evaluated change in cortisol curve features in individuals that participated in MESA Stress 1 and II CAR and daily cortisol slope became more blunted with aging [30], regardless of type 2 diabetes status. Our current results suggest that there may not be an additional type 2 diabetes effect beyond the aging effect. Another possibility is that if the effect of type 2 diabetes on cortisol dynamics is immediate (e.g., due to the metabolic milieu of insulin resistance, inflammation, etc.) and if this environment is equally present at both time points, then no association of type 2 diabetes with change in cortisol measures would be expected. We performed analyses of the cross-sectional association of type 2 diabetes status with cortisol curve features at the MESA Stress 2 visit and the findings were very similar to those in MESA Stress 1—(a) type 2 diabetes was associated with a blunted CAR in both sexes and (b) type 2 diabetes status was associated with less steep early decline slope and greater cortisol AUC in women and no association in men (data not shown). Type 2 diabetes status was not associated with wake-up cortisol or late decline slope, as in MESA Stress 1 (data not shown). In contrast to our cross-sectional findings at MESA Stress 1 and 2, we did not observe sex-specific effects in our longitudinal analysis.
The cross-sectional nature of the prior studies may account for the inconsistency in findings as there may be residual confounding due to multiple factors (e.g., physiological psychological, and neuropeptide stimuli) that can affect HPA axis regulation not measured in current studies and reverse causation [47, 49–52]. A strength of the current study is that we were able to examine the longitudinal effect of type 2 diabetes on daily curve features to determine the temporality of the association. In addition, in contrast to prior studies that did not adjust for a range of confounders [49] and included primarily Caucasian participants [50, 52], our study population was multiethnic and we were able to adjust for a large number of potential confounding factors.
Our study did have some limitations. We had repeated cortisol values for approximately half of the individuals who participated in the original MESA Stress I cohort. Included individuals younger compared to excluded individuals only enrolled in MESA Stress I, raising the possibility of survival bias. Although we were able to ultimately include 580 participants with repeated cortisol measures over time, other cross-sectional studies have included a larger number of participants [50, 52]. This raises the possibility that we may have been underpowered to identify statistically significant differences in change in cortisol features over time by type 2 diabetes status [30]. Although we were able to examine longitudinal change in cortisol curve features in individuals with and without type 2 diabetes over 6 years, perhaps this follow-up time period was not sufficient to identify potentially meaningful changes. Diabetes complications usually occur 5–10 years after the diagnosis and perhaps HPA axis dysregulation may require more time to develop. In the current study we could not rule in or rule out the presence of subclinical hypercortisolism as no other functional studies of the HPA axis were performed (e.g., 1 mg dexamethasone suppression test, 24 h urine-free cortisol). Additionally, the diagnosis of type 2 diabetes mellitus was made by a single fasting glucose greater than 126 mg/dL and that was not repeated. We also did not have accurate data on diabetes duration and lacked data on glycemic control (e.g., HbA1c) at the MESA Stress I visit, hypoglycemia frequency, and diabetes complications, all potentially important confounders in these associations. Finally, in MESA Stress II we only had self-reported wake-up time. While our subsidiary analysis suggests that wake-up time is an important variable in this association, additional studies with more accurate wake-up time measures are needed to confirm this pattern.
Conclusions
In summary our study is the first longitudinal study to examine the association of type 2 diabetes status with long-term changes in daily cortisol curve features. We did not identify any statistically significant differences in change in cortisol curve features by diabetes status. Larger cohort studies with repeated cortisol measures and longer follow-up are needed in order to further study the potential role of type 2 diabetes in HPA axis dysregulation.
Supplementary Material
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Funding MESA was supported by contracts NO1-HC-95159 through NO1-HC-95165 and NO1-HC-95169 from the National Heart, Lung, and Blood Institute (PI: ADR). MESA Stress Study was supported by RO1 HL10161-01A1 and R21 DA024273 (PI: ADR). EKS was supported by an institutional training grant from the National Institute of Diabetes, Digestive, and Kidney Diseases (T32DK062707).
Appendix 1
Let yijdk be the kth measure of cortisol of subject i at MESA Stress study j (j = 1, 2) on day d. The piecewise linear mixed effect model was specified as follows:
where βli = βl + bli, l = 0, 1, 3, 4, 5, 7 and b0i, b1i, b3i, b4i, b5i, b7i are individual-level random intercept and slope for individual i;
Timeij is the time (years) since the baseline study (MESA Stress I) for individual i at study j. Note that Timei1 = 0; tijdk is the time (h) since wake-up when the cortisol sample yijdk was collected;
Covi represents a set of sociodemographic factors and health-related factors for individual i at baseline study; Covi is excluded in Model 0 as the model is for an unadjusted analysis; Covi includes sociodemographic factors including age, sex, race/ethnicity, and socioeconomic status in Model 1; and additionally includes waist circumference, depressive symptoms, smoking status, and medication usage (beta-blocker, aspirins, inhaled or oral steroids, and hormone replacement therapy) in Model 2. Also, all covariates included in Covi are centered at their population average in the analysis; therefore, the estimates on the difference in cortisol feature change over time for each diabetes groups and the difference between groups (as shown in Supplementary Table 1) are interpreted at the population average, i.e., average level of sociodemographic characteristics and health-related factors.
Diabi is a binary variable indicating individual’s diabetes status (1: diabetes; 0: non-diabetes); eijdk is the unexplained deviation from the mean for the kth cortisol measure on day d at MESA Stress study j for individual i.
The estimates of the coefficients for the terms that involve diabetes status were used to derive estimates of the cortisol features by diabetes groups, and the difference in the change of daily cortisol features over time between diabetes groups, as shown in Supplementary Table 1.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s12020-016-0887-8) contains supplementary material, which is available to authorized users.
Conflict of Interest The authors have no relevant conflict of interest to disclose.
Compliance with ethical standards
Ethical Standards All procedures performed in the study were in accordance with the ethical standards of the institutional and/or national committees and have been performed in accordance with the ethical standards as laid down in the 1964 Helsinki declaration and its latter amendments or comparable ethical standards.
Informed Consent All participants provided informed consent and the above studies were approved by the Institutional Review Boards of each institution.
References
- 1.Peeke PM, Chrousos GP. Hypercortisolism and obesity. Ann. N. Y. Acad. Sci. 1995;771:665–676. doi: 10.1111/j.1749-6632.1995.tb44719.x. [DOI] [PubMed] [Google Scholar]
- 2.Mazziotti G, Gazzaruso C, Giustina A. Diabetes in cushing syndrome: basic and clinical aspects. TEM. 2011;22(12):499–506. doi: 10.1016/j.tem.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Anagnostis P, Athyros VG, Tziomalos K, Karagiannis A, Mikhailidis DP. Clinical review: the pathogenetic role of cortisol in the metabolic syndrome: a hypothesis. J. Clin. Endocrinol. Metab. 2009;94(8):2692–2701. doi: 10.1210/jc.2009-0370. [DOI] [PubMed] [Google Scholar]
- 4.Bruehl H, Rueger M, Dziobek I, Sweat V, Tirsi A, Javier E, Arentoft A, Wolf OT, Convit A. Hypothalamic-pituitary-adrenal axis dysregulation and memory impairments in type 2 diabetes. J. Clin. Endocrinol. Metab. 2007;92(7):2439–2445. doi: 10.1210/jc.2006-2540. [DOI] [PubMed] [Google Scholar]
- 5.Chiodini I, Torlontano M, Scillitani A, Arosio M, Bacci S, Di Lembo S, Epaminonda P, Augello G, Enrini R, Ambrosi B, Adda G, Trischitta V. Association of subclinical hypercortisolism with type 2 diabetes mellitus: a case-control study in hospitalized patients. Eur. J. Endocrinol. 2005;153(6):837–844. doi: 10.1530/eje.1.02045. [DOI] [PubMed] [Google Scholar]
- 6.Godoy-Matos AF, Vieira AR, Moreira RO, Coutinho WF, Carraro LM, Moreira DM, Pasquali R, Meirelles RM. The potential role of increased adrenal volume in the pathophysiology of obesity-related type 2 diabetes. J. Endocrinol. Invest. 2006;29(2):159–163. doi: 10.1007/BF03344090. [DOI] [PubMed] [Google Scholar]
- 7.Champaneri S, Xu X, Carnethon MR, Bertoni AG, Seeman T, DeSantis AS, Diez Roux A, Shrager S, Golden SH. Diurnal salivary cortisol is associated with body mass index and waist circumference: the multiethnic study of atherosclerosis. Obesity. 2013;21(1):E56–E63. doi: 10.1002/oby.20047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosmond R, Wallerius S, Wanger P, Martin L, Holm G, Bjorntorp P. A 5-year follow-up study of disease incidence in men with an abnormal hormone pattern. J. Intern. Med. 2003;254(4):386–390. doi: 10.1046/j.1365-2796.2003.01205.x. [DOI] [PubMed] [Google Scholar]
- 9.Gungunes A, Sahin M, Demirci T, Ucan B, Cakir E, Arslan MS, Unsal IO, Karbek B, Caliskan M, Ozbek M, Cakal E, Delibasi T. Cushing’s syndrome in type 2 diabetes patients with poor glycemic control. Endocrine. 2014;47(3):895–900. doi: 10.1007/s12020-014-0260-8. [DOI] [PubMed] [Google Scholar]
- 10.Catargi B, Rigalleau V, Poussin A, Ronci-Chaix N, Bex V, Vergnot V, Gin H, Roger P, Tabarin A. Occult Cushing’s syndrome in type-2 diabetes. J. Clin. Endocrinol. Metab. 2003;88(12):5808–5813. doi: 10.1210/jc.2003-030254. [DOI] [PubMed] [Google Scholar]
- 11.Wei L, MacDonald TM, Walker BR. Taking glucocorticoids by prescription is associated with subsequent cardiovascular disease. Ann. Intern. Med. 2004;141(10):764–770. doi: 10.7326/0003-4819-141-10-200411160-00007. [DOI] [PubMed] [Google Scholar]
- 12.Souverein PC, Berard A, Van Staa TP, Cooper C, Egberts AC, Leufkens HG, Walker BR. Use of oral glucocorticoids and risk of cardiovascular and cerebrovascular disease in a population based case-control study. Heart. 2004;90(8):859–865. doi: 10.1136/hrt.2003.020180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Etxabe J, Vazquez JA. Morbidity and mortality in Cushing’s disease: an epidemiological approach. Clin. Endocrinol. 1994;40(4):479–484. doi: 10.1111/j.1365-2265.1994.tb02486.x. [DOI] [PubMed] [Google Scholar]
- 14.Neary NM, Booker OJ, Abel BS, Matta JR, Muldoon N, Sinaii N, Pettigrew RI, Nieman LK, Gharib AM. Hypercortisolism is associated with increased coronary arterial atherosclerosis: analysis of noninvasive coronary angiography using multidetector computerized tomography. J. Clin. Endocrinol. Metab. 2013;98(5):2045–2052. doi: 10.1210/jc.2012-3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Champaneri S, Wand GS, Malhotra SS, Casagrande SS, Golden SH. Biological basis of depression in adults with diabetes. Curr. Diab. Rep. 2010;10(6):396–405. doi: 10.1007/s11892-010-0148-9. [DOI] [PubMed] [Google Scholar]
- 16.Szmitko PE, Wang CH, Weisel RD, de Almeida JR, Anderson TJ, Verma S. New markers of inflammation and endothelial cell activation: part I. Circulation. 2003;108(16):1917–1923. doi: 10.1161/01.CIR.0000089190.95415.9F. [DOI] [PubMed] [Google Scholar]
- 17.Ridker PM, Buring JE, Shih J, Matias M, Hennekens CH. Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation. 1998;98(8):731–733. doi: 10.1161/01.cir.98.8.731. [DOI] [PubMed] [Google Scholar]
- 18.Pruessner JC, Wolf OT, Hellhammer DH, Buske-Kirschbaum A, von Auer K, Jobst S, Kaspers F, Kirschbaum C. Free cortisol levels after awakening: a reliable biological marker for the assessment of adrenocortical activity. Life Sci. 1997;61(26):2539–2549. doi: 10.1016/s0024-3205(97)01008-4. [DOI] [PubMed] [Google Scholar]
- 19.Kirschbaum C, Hellhammer DH. Salivary cortisol in psychobiological research: an overview. Neuropsychobiology. 1989;22(3):150–169. doi: 10.1159/000118611. [DOI] [PubMed] [Google Scholar]
- 20.Karlamangla AS, Friedman EM, Seeman TE, Stawksi RS, Almeida DM. Daytime trajectories of cortisol: demographic and socioeconomic differences—findings from the National Study of Daily Experiences. Psychoneuroendocrinology. 2013;38(11):2585–2597. doi: 10.1016/j.psyneuen.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sephton SE, Sapolsky RM, Kraemer HC, Spiegel D. Diurnal cortisol rhythm as a predictor of breast cancer survival. J. Natl. Cancer Inst. 2000;92(12):994–1000. doi: 10.1093/jnci/92.12.994. [DOI] [PubMed] [Google Scholar]
- 22.Adam EK, Gunnar MR. Relationship functioning and home and work demands predict individual differences in diurnal cortisol patterns in women. Psychoneuroendocrinology. 2001;26(2):189–208. doi: 10.1016/s0306-4530(00)00045-7. [DOI] [PubMed] [Google Scholar]
- 23.Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: potential indices of risk in human development. Dev. Psychopathol. 2001;13(3):515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- 24.Kunz-Ebrecht SR, Kirschbaum C, Marmot M, Steptoe A. Differences in cortisol awakening response on work days and weekends in women and men from the Whitehall II cohort. Psychoneuroendocrinology. 2004;29(4):516–528. doi: 10.1016/s0306-4530(03)00072-6. [DOI] [PubMed] [Google Scholar]
- 25.Wright CE, Steptoe A. Subjective socioeconomic position, gender and cortisol responses to waking in an elderly population. Psychoneuroendocrinology. 2005;30(6):582–590. doi: 10.1016/j.psyneuen.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 26.Cohen S, Schwartz JE, Epel E, Kirschbaum C, Sidney S, Seeman T. Socioeconomic status, race, and diurnal cortisol decline in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Psychosom. Med. 2006;68(1):41–50. doi: 10.1097/01.psy.0000195967.51768.ea. [DOI] [PubMed] [Google Scholar]
- 27.Saxbe DE, Repetti RL, Nishina A. Marital satisfaction, recovery from work, and diurnal cortisol among men and women. Health Psychol. 2008;27(1):15–25. doi: 10.1037/0278-6133.27.1.15. [DOI] [PubMed] [Google Scholar]
- 28.Hajat A, Diez-Roux A, Franklin TG, Seeman T, Shrager S, Ranjit N, Castro C, Watson K, Sanchez B, Kirschbaum C. Socioeconomic and race/ethnic differences in daily salivary cortisol profiles: the multi-ethnic study of atherosclerosis. Psychoneuroendocrinology. 2010;35(6):932–943. doi: 10.1016/j.psyneuen.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumari M, Shipley M, Stafford M, Kivimaki M. Association of diurnal patterns in salivary cortisol with all-cause and cardiovascular mortality: findings from the Whitehall II study. J. Clin. Endocrinol. metab. 2011;96(5):1478–1485. doi: 10.1210/jc.2010-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang X, Sanchez BN, Golden SH, Shrager S, Kirschbaum C, Karlamangla AS, Seeman TE, Roux AV. Stability and predictors of change in salivary cortisol measures over six years: MESA. Psychoneuroendocrinology. 2014;49:310–320. doi: 10.1016/j.psyneuen.2014.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Golden SH, Sanchez BN, Wu M, Champaneri S, Diez Roux AV, Seeman T, Wand GS. Relationship between the cortisol awakening response and other features of the diurnal cortisol rhythm: the Multi-Ethnic Study of Atherosclerosis. Psychoneuroendocrinology. 2013;38(11):2720–2728. doi: 10.1016/j.psyneuen.2013.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Golden SH, Wand GS, Malhotra S, Kamel I, Horton K. Reliability of hypothalamic-pituitary-adrenal axis assessment methods for use in population-based studies. Eur. J. Epidemiol. 2011;26(7):511–525. doi: 10.1007/s10654-011-9585-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tirabassi G, Boscaro M, Arnaldi G. Harmful effects of functional hypercortisolism: a working hypothesis. Endocrine. 2014;46(3):370–386. doi: 10.1007/s12020-013-0112-y. [DOI] [PubMed] [Google Scholar]
- 34.Joseph JJ, Wang X, Spanakis E, Seeman T, Wand G, Needham B, Golden SH. Diurnal salivary cortisol, glycemia and insulin resistance: the multi-ethnic study of atherosclerosis. Psychoneuroendocrinology. 2015;62:327–335. doi: 10.1016/j.psyneuen.2015.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-ethnic study of atherosclerosis: objectives and design. Am. J. Epidemiol. 2002;156(9):871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 36.Raff H. Update on late-night salivary cortisol for the diagnosis of Cushing’s syndrome: methodological considerations. Endocrine. 2013;44(2):346–349. doi: 10.1007/s12020-013-0013-0. [DOI] [PubMed] [Google Scholar]
- 37.Bellastella G, Maiorino MI, De Bellis A, Vietri MT, Mosca C, Scappaticcio L, Pasquali D, Esposito K, Giugliano D. Serum but not salivary cortisol levels are influenced by daily glycemic oscillations in type 2 diabetes. Endocrine. 2015 doi: 10.1007/s12020-015-0777-5. [DOI] [PubMed] [Google Scholar]
- 38.Hill Golden S, Sanchez BN, Desantis AS, Wu M, Castro C, Seeman TE, Tadros S, Shrager S, Diez Roux AV. Salivary cortisol protocol adherence and reliability by socio-demographic features: the Multi-Ethnic Study of Atherosclerosis. Psychoneuroendocrinology. 2014;43:30–40. doi: 10.1016/j.psyneuen.2014.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hellhammer DH, Wust S, Kudielka BM. Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology. 2009;34(2):163–171. doi: 10.1016/j.psyneuen.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 40.Yeh KC, Kwan KC. A comparison of numerical integrating algorithms by trapezoidal, Lagrange, and spline approximation. J. Pharmacokinet. Biopharm. 1978;6(1):79–98. doi: 10.1007/BF01066064. [DOI] [PubMed] [Google Scholar]
- 41.Badrick E, Kirschbaum C, Kumari M. The relationship between smoking status and cortisol secretion. J. Clin. Endocrinol. metab. 2007;92(3):819–824. doi: 10.1210/jc.2006-2155. [DOI] [PubMed] [Google Scholar]
- 42.Bertoni AG, Whitt-Glover MC, Chung H, Le KY, Barr RG, Mahesh M, Jenny NS, Burke GL, Jacobs DR. The association between physical activity and subclinical atherosclerosis: the Multi-Ethnic Study of Atherosclerosis. Am. J. Epidemiol. 2009;169(4):444–454. doi: 10.1093/aje/kwn350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Radloff L. The CES-D scale: a self-report depression scale for research in the general population. Appl. Psychol. Meas. 1977;1:385–401. [Google Scholar]
- 44.Pilkonis PA, Imber SD, Rubinsky P. Dimensions of life stress in psychiatric patients. J. Human Stress. 1985;11(1):5–10. doi: 10.1080/0097840X.1985.9936732. [DOI] [PubMed] [Google Scholar]
- 45.Mujahid MS, Diez Roux AV, Cooper RC, Shea S, Williams DR. Neighborhood stressors and race/ethnic differences in hypertension prevalence (the Multi-Ethnic Study of Atherosclerosis) Am. J. Hypertens. 2011;24(2):187–193. doi: 10.1038/ajh.2010.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adam EK, Kumari M. Assessing salivary cortisol in largescale, epidemiological research. Psychoneuroendocrinology. 2009;34(10):1423–1436. doi: 10.1016/j.psyneuen.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 47.Champaneri S, Xu X, Carnethon MR, Bertoni AG, Seeman T, Diez Roux A, Golden SH. Diurnal salivary cortisol and urinary catecholamines are associated with diabetes mellitus: the Multi-Ethnic Study of Atherosclerosis. Metabolism. 2012;61(7):986–995. doi: 10.1016/j.metabol.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Casella G, Berger R. Statistical Inference. Vol. 2. Pacific Grove: Duxbury Press; 2001. [Google Scholar]
- 49.Bruehl H, Wolf OT, Convit A. A blunted cortisol awakening response and hippocampal atrophy in type 2 diabetes mellitus. Psychoneuroendocrinology. 2009;34(6):815–821. doi: 10.1016/j.psyneuen.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lederbogen F, Hummel J, Fademrecht C, Krumm B, Kuhner C, Deuschle M, Ladwig KH, Meisinger C, Wichmann HE, Lutz H, Breivogel B. Flattened circadian cortisol rhythm in type 2 diabetes. Exp. Clin. Endocrinol. Diabet. 2011;119(9):573–575. doi: 10.1055/s-0031-1275288. [DOI] [PubMed] [Google Scholar]
- 51.Vreeburg SA, Kruijtzer BP, van Pelt J, van Dyck R, DeRijk RH, Hoogendijk WJ, Smit JH, Zitman FG, Penninx BW. Associations between sociodemographic, sampling and health factors and various salivary cortisol indicators in a large sample without psychopathology. Psychoneuroendocrinology. 2009;34(8):1109–1120. doi: 10.1016/j.psyneuen.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 52.Hackett RA, Steptoe A, Kumari M. Association of diurnal patterns in salivary cortisol with type 2 diabetes in the Whitehall II study. J. Clin. Endocrinol. metab. 2014 doi: 10.1210/jc.2014-2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.