Abstract
Abdominal pain is a chronic condition experienced by approximately 20% of individuals in the United States. The purpose of the study was to assess the validity of the Gastrointestinal Pain Pointer as a measure of abdominal pain intensity. A prospective longitudinal time-series study design was utilized. The sample included 93 outpatients (58.1% female). Participants met Rome III criteria for irritable bowel syndrome (n = 32) or were healthy controls (n = 61). The Gastrointestinal Pain Pointer, a new electronic pain assessment tool, was used to assess self-reported abdominal pain intensity among participants before and after ingestion of an intestinal permeability test solution across 11 time points over a 5-hour time period. The results were compared with the Short-Form McGill Pain Questionnaire. The Gastrointestinal Pain Pointer was found to be valid in the assessment of abdominal pain intensity. The tool is a novel and valid measure of abdominal pain intensity that enhances the ability for clinicians to better quantify, in real time, patient-related pain outcomes for both clinical care and research.
Up to 20% of the United States (U.S.) population reports digestive symptoms (Grundmann & Yoon, 2010), and abdominal pain is the most common gastrointestinal (GI) diagnosis for outpatient visits (Sandler et al., 2002). Abdominal pain has been universally endorsed by those who suffer from irritable bowel syndrome (IBS) (Drossman et al., 2009). For persons with IBS or other digestive disorders, chronic abdominal pain disrupts daily life and negatively impacts quality of life (Monnikes, 2011).
Background
In chronic abdominal pain, descriptors of the experience include cramping, aching, discomfort, or specifically abdominal pain (Katz & Melzack, 2011). Although other studies have assessed abdominal pain through subjective patient response questionnaires (Abernethy et al., 2010; Adam, Liebregts, Saadat-Gilani, Vinson, & Holtmann, 2005; Bengtsson, Ohlsson, & Ulander, 2007; Berger, Damico, Menees, Fenner, & Haefner, 2012; Betz, Mannsdorfer, & Bischoff, 2013; Kendall et al., 2013; Kovacic, Williams, Li, Chelimsky, & Miranda, 2013; Malaty et al., 2005; Mohammad et al., 2013; Ramaswami et al., 2012; Rentz et al., 2004; Talley, Boyce, Owen, Newman, & Paterson, 1995; Wiklund et al., 2003; Yacob et al., 2013), pain assessment persists to be challenging (Drossman et al., 2009), with studies suggesting that clinicians have difficultly both assessing and documenting changes in pain over time (Drossman et al., 2006). Research on pain in the U.S. has been encouraged through the U.S. Public Health Service Act (2010) and through the development of the Interagency Pain Research Coordinating Committee (U.S. Public Health Service Act, 2010). Moreover, The Joint Commission has developed specific standards for the care of individuals with pain (U.S. Public Health Service Act, 2010). These standards include the patient's right to appropriate assessment of the nature and intensity of pain, documentation of pain at regular intervals, education of patients with pain and their family, and follow-up care.
Electronic pain assessment tools have been developed to address these mandates. In particular, various tools have been developed to measure pain intensity including blinding features, language descriptors, body model/image, spatial pain indicator and physiologic pain (Morren, van Dulmen, Ouwerkerk, & Bensing, 2009; Stinson, 2009). Despite these developments, none of the currently available tools have alerting mechanisms for pain assessment reminders, nor an option to choose body gender or type such as normal weight or overweight images. Therefore, we developed the Gastrointestinal Pain Pointer (GIPP) to address these unmet needs. The GIPP is a novel, electronic tool designed to assess in real-time self-reported abdominal pain.
The goal of this study was to assess the validity of the GIPP in real time, with subjective measurement of GI symptoms, specifically abdominal pain intensity, in two groups: participants with chronic abdominal pain (IBS) and healthy controls after ingestion of a test solution (Del Valle-Pinero et al., 2013) that potentially reproduced abdominal pain symptoms. These two groups allowed for a greater range of pain intensity scores for which to validate the GIPP. Ratings on the GIPP were compared with the Short-Form McGill Pain Questionnaire (SF-MPQ) ratings to evaluate convergent validity.
Methods
Patient Population
The study was approved by the institutional review board (2009) and all participants provided informed consent prior to participation in the study. Patients reporting chronic abdominal pain (pain group) and healthy controls, hence forward referred to as participants, were recruited between 2009 and 2013 primarily from the metropolitan Washington D.C. area. The study was conducted at the National Institutes of Health Clinical Center in Bethesda, Maryland (Trial Registration: http://www.clinicaltrials.gov, NCT 00824941).
Inclusion criteria for the pain group included a history of chronic abdominal pain defined as a self-reported abdominal pain for greater than 6 months and met Rome III criteria for IBS (Drossman et al., 2006). The control group reported no chronic abdominal pain. Participants from both groups were excluded if they had a history of an organic GI disease (e.g., inflammatory bowel disease, celiac disease, biliary disorders, bowel resection); cardiac, pulmonary, neurologic, renal, endocrine, or gynecologic pathology, severe comorbid pain (e.g., fibromyalgia), or a psychiatric condition (e.g., bipolar or psychotic disorder) as these may impact pain pathways. Additional exclusions were as follows: daily medications for GI symptoms; medications that might alter serotonin (e.g., serotonin specific reuptake inhibitors); catecholamines (e.g., tricycle antidepressants but not inhaled β-agonist for mild-moderate asthma); inability to physically use a touch screen; visual impairment; or current institutionalization.
Instruments
Gastrointestinal Pain Pointer
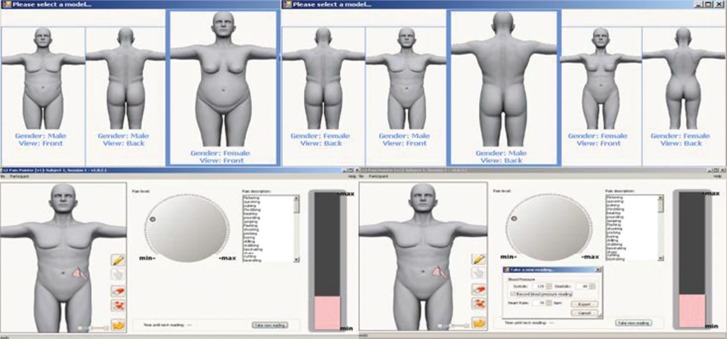
The GIPP tool allows the participant to identify pain intensity via a dial-up electronic interface with 0–100 scaled data capture without numeric quantification visible to the participant. The GIPP also captures pain location and pain word descriptors, as well as physiologic data such as heart rate and blood pressure. Participants self-administer the GIPP with the use of a graphical interface to choose their gender (male/female) and body type (normal or overweight), record the location and intensity of the pain, and choose words to describe the pain (Figure 1). The clinician simultaneously captures heart rate and blood pressure either manually or through the assistance of an electronic device. Data are captured and time is automatically entered into an electronic storage file in real time for analysis. The binary and executable code was developed and processed by Psychology Software Tools, Inc. For the purposes of this study, only the pain intensity rating was used for the analysis.
FIGURE 1.
The Gastrointestinal Pain Pointer. Developed by Henderson & Zuccolotto, Public Health Service Invention #E-175-2010.
The Short-Form McGill Pain Questionnaire
The SF-MPQ is a multidimensional measure of perceived pain that was used to assess pain intensity (Hawker, Mian, Kendzerska, & French, 2011; Katz & Melzack, 2011). The SF-MPQ was chosen to reduce participant burden as it takes 2–5 minutes to complete (Hawker et al., 2011). The pain intensity ratings were obtained from the visual analog scale portion of the SF-MPQ. The scores were assigned by location along a continuous line (Figure 2), according to published procedures (Katz & Melzack, 2011). The word “descriptors” was not included in this analysis as the focus of the study was to validate pain intensity. In addition, the GIPP includes specific GI symptom descriptors (e.g., “bloating”) that are not available in the SF-MPQ; therefore, a comparison of word descriptors was not applicable.
FIGURE 2.
McGill Pain Questionnaire, example of marked and measured visual analog pain intensity rating scale. © Copyright 1984. Reprinted with permission from Dr. R. Melzack.
Study Design and Data Collection
After an initial outpatient screening visit that included history, physical examination, and laboratory evaluation, participants who met inclusion criteria were invited back for a second outpatient visit at the Clinical Research Center of the National Institutes of Health. During the second visit, participants' (including controls') abdominal pain intensity, location, and descriptors of pain using both the GIPP and the SF-MPQ were assessed before and after ingestion of a 100-ml intestinal permeability test solution per published recommendations (Del Valle-Pinero et al., 2013) that may induce abdominal pain symptoms. Self-administered GIPP and SF-MPQ were simultaneously recorded at 11 time points (30 minutes before ingesting the solution, at the time of ingestion, and at 15-, 30-, 45-, 60-, 90-, 120-, 180-, 240-, and 300-minute intervals postingestion to capture pain intensity over time).
Statistical Analysis
Descriptive statistics, general linear mixed models, Pearson product–moment correlations, pooled within-sample correlational analysis, between-sample correlation (pain group–no pain group), and intraclass correlation coefficient were used for statistical analysis (SPSS version 15, Chicago, IL). Convergent validity was assessed by the use of correlation analysis between the GIPP and SF-MPQ VAS scale scores at each point period using pooled within-sample correlational analysis. Predictive validity was assessed by evaluating the ability of the GIPP to discriminate between the pain group and the control group using between-sample correlations. Intraclass correlation coefficients for the pain group versus control group were used to evaluate the GIPP for consistency over time compared with the SF-MPQ. The investigators had access to the de-identified study data and reviewed and approved the final manuscript.
Results
Sample Characteristics
A total sample of 93 participants (58.1% female; 27.9 ± 7.7 years of age, 50.5% non-Hispanic White) completed the study (Table 1). There were 32 participants in the pain group (IBS) and 61 in the control group.
TABLE 1. Sample Demographics.
| Variable | Overall (N = 93) | Pain Group (n = 32) | Control Group (n = 61) |
|---|---|---|---|
| Gender, n (%) | |||
| Male | 39 (41.9) | 10 (31.3) | 29 (47.5) |
| Female | 54 (58.1) | 22 (68.8) | 32 (52.5) |
| Race, n (%) | |||
| Caucasian | 47 (50.5) | 17 (53.1) | 30 (49.2) |
| African American/Black | 25 (26.9) | 10 (31.3) | 15 (24.6) |
| Asian | 14 (15.1) | 3 (9.4) | 11 (18.0) |
| Other | 7 (7.5) | 2 (6.3) | 5 (8.2) |
| Age, M (SD) | 27.9 ± 7.8 | 26.8 ± 7.1 | 28.5 ± 8.2 |
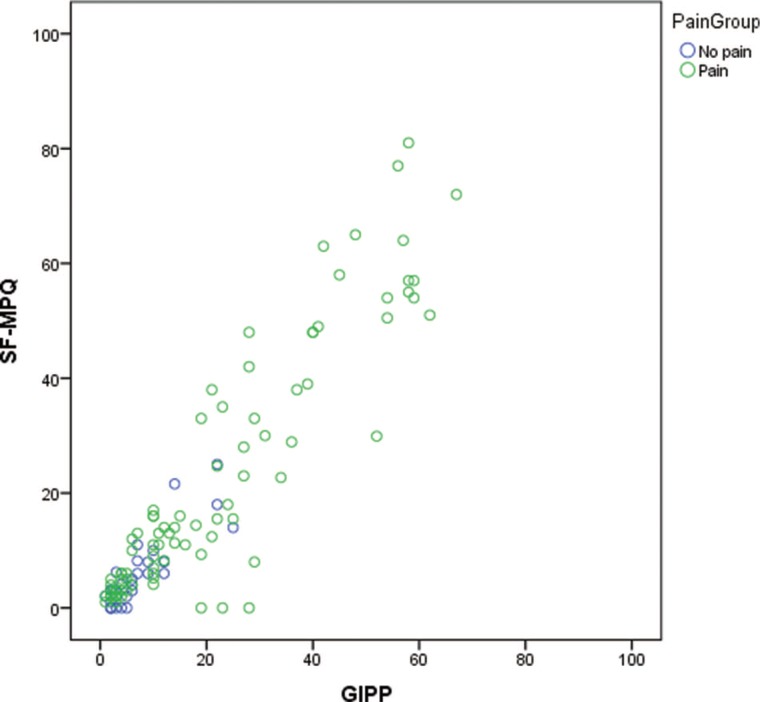
The pooled within-subjects correlation between the SF-MPQ and the GIPP was .80 (p ≤ .001) for the total sample. The between-sample correlation was .86 (p ≤ .001); however, when controlling for participants who reported pain as “bloating” on the GIPP but was not an option as a descriptor on the SF-MPQ, the between-sample correlation was .93 (p ≤ .001) (Figure 3). The intraclass correlation coefficient was .57 for the SF-MPQ and .49 for the GIPP. For the control group, the pooled within-subjects correlation between SF-MPQ and GIPP was .77 (p ≤ .001) and the between-sample correlation was .13 (p = .319). The intraclass correlation coefficient was .84 for the SF-MPQ and .27 for the GIPP. For the pain group, the pooled within-subjects correlation between SF-MPQ and GIPP was .80 (p ≤ .001) and the between-sample correlation was .98 (p ≤ .001). The intraclass correlation coefficient was .48. for the SF-MPQ and .46 for the GIPP.
FIGURE 3.
Scatterplot of GIPP by SF-MPQ pain intensity scores. GIPP = Gastrointestinal Pain Pointer; SF-MPQ = Short-Form McGill Pain Questionnaire.
A mixed analysis of variance was performed on pain as a function of group (pain, control), time, and measure (GIPP, SF-MPQ). Within-subjects independent variables were time (11 levels) and measure. The between-subjects independent variable was group membership as pain group compared with the control group. Because the distribution of data was not normal, a bootstrap-adjusted ANOVA was also performed. There was no significant difference on pain between measures averaged across time and group, F(1, 82) = 2.25, bootstrap adjusted p = .208, p = .138, η2p =.027 [main effect of measures]. The pattern of difference on pain between GIPP and SF-MPQ measures was not significantly different between pain and no-pain groups, F(1, 82) = .19, bootstrap adjusted p = .678, p = .666, η2p =.002 [interaction effect of measure by group]. There was no significant difference on pain among time averaged across measures and group, F(10, 820) = 1.74, bootstrap adjusted p = .585, p = .067, η2p =.021 [main effect of time]. The pattern of difference on pain among time was not significantly different between pain and control groups, F(10, 820) = 1.52, bootstrap adjusted p = .653, p = .126, η2p =.018 [interaction effect of time by group]. The pattern of difference on pain among time was not significantly different between GIPP and SF-MPQ measures, F(10, 82) = 2.02, bootstrap adjusted p = .516, p = .029, η2p =.024 [interaction effect of time by measure]. The interaction effect of time by group on pain was not significantly different between GIPP and SF-MPQ measures, F(10, 820) = 1.34, bootstrap adjusted p = .694, p = .203, η2p =.016 [three-way interaction effect of measure by time by group]. The GIPP and SF-MFQ were not significantly different within the pain (GIPP: M = 6.35, SE = 0.77; SF-MFQ: M = 6.67, SE = 0.85) and no-pain groups (GIPP: M = 0.36, SE = 0.08; SF-MFQ: M = 0.89, SE = 0.19). The pain group (M = 4.67, SE = 0.75) had significantly higher pain scores than the no-pain group (M = 0.63, SE = 0.47) averaged across time and measure, F(1, 82) = 20.98, bootstrap adjusted p = .012, p < .001, η2p =.204 [main effect of group].
Discussion
Our study demonstrated that the GIPP, an electronic pain assessment tool, effectively measured pain intensity among participants over 11 time points when compared with the SF-MPQ. The most important requirement of a pain assessment tool is that it meets certain requirements to be valid, reliable, consistent, and useful (Katz & Melzack, 2011). The uniqueness of the GIPP is that it meets mandatory reporting requirements and is a point of care, patient-friendly, easy-to-use electronic format to assess pain. When compared to ratings made on the SF-MPQ at the same time points, we found that GIPP scores were consistent with the SF-MPQ scores, and that there were no significant differences in the ratings of pain for either the pain group or the control group when using the GIPP. Such findings demonstrated that the GIPP was a valid tool as it was able to produce results similar to the SF-MPQ over time.
There was a high correlation on pain ratings for GIPP versus SF-MPQ across all participants and between results reported for the GIPP and the SF-MPQ. Within each group, pain ratings continued to be similar, although somewhat less than for all participants combined. Overall, results demonstrated that ratings on the GIPP mirrored pain ratings entered by participants using the marked line designation for the SF-MPQ. The no-pain group also demonstrated similarity in pain ratings when using the GIPP and the SF-MPQ. Pain ratings, using the SF-MPQ, were similar within the group of participants with no pain. However, this within-group similarity for pain ratings was modest only when reported using the GIPP. Although there were fewer participants who reported pain compared with the no-pain control group, ratings for the pain group were consistent across the GIPP and the SF-MPQ for pain intensity.
The GIPP demonstrated its utility as a valid tool for the assessment of abdominal pain intensity as an indicator of GI symptoms. The GIPP has practical nursing application in both clinical care and research settings. Its novel and additional features include a “bloating” descriptor that provides participants with expanded options for describing their GI symptoms. This additional descriptor may assist healthcare providers with more effective treatment and management for GI symptoms. Another feature demonstrated by the GIPP is its time-saving ability to provide real time, heart rate, and blood pressure readings that may be simultaneously imported into the medical record through the GIPP's electronic interface. The GIPP also has potential practical use with nonverbal individuals in various healthcare settings and may be translated into different languages.
This study has some limitations. We tested the GIPP in a limited cohort of participants with and without IBS. Additional testing of the tool in individuals with other chronic conditions should be carried out to further assess the validity and reliability of the tool. Moreover, we included a relatively young population of individuals; therefore, further studies are needed to validate the GIPP in other age cohorts. Gastrointestinal symptoms and abdominal pain may vary depending on age, including children, aging individuals, postmenopausal women, and individuals living with comorbidities.
Noting the aforementioned limitations, pain is not a one-dimensional experience, particularly for patients suffering from chronic GI symptoms. With regard to IBS, clinicians' understanding of the complexity of the patient's experience with GI symptoms is difficult (Drossman et al., 2009). Therefore, tools such as the GIPP are valuable for use in both clinical care and research settings.
There continues to be an unmet clinical and research need for expanded clinical assessment of GI symptoms and improved characterization of abdominal pain phenotypes that include a real-time assessment of both subjective (patient-reported) and objective (measureable and quantifiable) pain. Furthermore, because the GIPP is able to characterize pain intensity, location, and qualitative description, it can potentially be utilized in various diseases, particularly in those who have no clearly defined etiology. As such, the GIPP is a valuable asset and adds a unique contribution to patient-reported outcome of GI pain and discomfort assessment.
Summary
In summary, the GIPP is a valid electronic pain assessment tool that differs from other available measures. Unique features of the GIPP include the ability to (1) record objective measures such as blood pressures and heart rates, (2) alert a user about when to collect subsequent subjective pain measurement data and/or objective measure data, and (3) select a body type to display. Thus, the GIPP is a unique, valid tool that may improve the assessment of abdominal pain and discomfort.
ACKNOWLEDGMENTS
The protocol (09-NR-0064) was approved by the Institutional Review Board at the National Institutes of Health. (Clinicaltrial.gov # NCT00824941).
We thank the National Institutes of Health (NIH), Division of Intramural Research, National Institute of Nursing Research (NINR) for the support to W.A.H., 1ZIANR000018-01-05; National Institute on Minority Health & Health Disparities NIH fellowship DREAM award to B.R.W., 1K22MD006143-01; NIH Intramural Research Training Awards to L.B.S., S.K.A., and N.H.F., Department of Health and Human Services, Bethesda, Maryland. Additional support was provided via Material Transfer Agreement with Psychology Software Tools, Inc., for software development and binary/executable format of the Gastrointestinal Pain Pointer (GIPP) software. Support was provided by Dr. Robert Shulman for the intestinal permeability formulation, Dr. R. Melzack for use of the Short-Form McGill Pain Questionnaire, Dr. Ann Berger and the staff of the Clinical Center of the NIH, Dr. Kong Chen and the NIDDK Metabolic Program of Care, and the University of Pittsburgh School of Nursing for use of the Socio-demographic Questionnaire (1999); Center for Research in Chronic Disorders (P30 NR003924).
The opinions expressed herein and the interpretation and reporting of these data are the responsibility of the author(s) and should not be seen as an official recommendation, interpretation, or policy of the National Institutes of Health.
Footnotes
The Gastrointestinal Pain Pointer is a research assessment tool and is not approved as a medical device. Employee Invention Report #NR-002, PHS E-175–2010 (Dr. Wendy A. Henderson) licensing available with Psychology Software Tools, Inc. Mr. Anthony Zuccolotto of Psychology Software Tools, Inc. discloses employee and stock ownership. Please note, Dr. Kevin H. Kim is deceased (2014), and the enclosed work and approval for publication of the manuscript occurred prior to his death. The remaining authors have no potential conflicts of interest to disclose.
REFERENCES
- Abernethy A. P., Zafar S. Y., Uronis H., Wheeler J. L., Coan A., Rowe K., Herndon J. E., II (2010). Validation of the Patient Care Monitor (Version 2.0): a review of system assessment instrument for cancer patients. Journal of Pain and Symptom Management, 40(4), 545–558. 10.1016/j.jpainsymman.2010.01.017. [DOI] [PubMed] [Google Scholar]
- Adam B., Liebregts T., Saadat-Gilani K., Vinson B., Holtmann G. (2005). Validation of the gastrointestinal symptom score for the assessment of symptoms in patients with functional dyspepsia. Alimentary Pharmacology & Therapeutics, 22(4), 357–363. 10.1111/j.1365-2036.2005.02572.x. [DOI] [PubMed] [Google Scholar]
- Bengtsson M., Ohlsson B., Ulander K. (2007). Development and psychometric testing of the Visual Analogue Scale for Irritable Bowel Syndrome (VAS-IBS). BMC Gastroenterology, 7, 16. 10.1186/1471-230X-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger M. B., Damico N. J., Menees S. B., Fenner D. E., Haefner H. K. (2012). Rates of self-reported urinary, gastrointestinal, and pain comorbidities in women with vulvar lichen sclerosus. Journal of Lower Genital Tract Disease, 16(3), 285–289. 10.1097/LGT.0b013e3182562f1e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz C., Mannsdorfer K., Bischoff S. C. (2013). [Validation of the IBS-SSS]. Z Gastroenterol, 51(10), 1171–1176. 10.1055/s-0033-1335260. [DOI] [PubMed] [Google Scholar]
- Del Valle-Pinero A. Y., Van Deventer H. E., Fourie N. H., Martino A. C., Patel N. S., Remaley A. T., Henderson W. A. (2013). Gastrointestinal permeability in patients with irritable bowel syndrome assessed using a four probe permeability solution. Clinica Chimica Acta, 418, 97–101. 10.1016/j.cca.2012.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drossman D. A., Chang L., Schneck S., Blackman C., Norton W. F., Norton N. J. (2009). A focus group assessment of patient perspectives on irritable bowel syndrome and illness severity. Digestive Diseases and Sciences, 54(7), 1532–1541. 10.1007/s10620-009-0792-6. [DOI] [PubMed] [Google Scholar]
- Drossman D. A., Corazziari E., Delvaux M., Spiller R. C., Talley N. J., Thompson W. G., Whitehead W. E. (2006). Rome III; The functional gastrointestinal disorders. McLean, VA: Degnon Associates. [Google Scholar]
- Grundmann O., Yoon S. L. (2010). Irritable bowel syndrome: Epidemiology, diagnosis and treatment: An update for health-care practitioners. Journal of Gastroenterology and Hepatology, 25(4), 691–699. 10.1111/j.1440-1746.2009.06120.x. [DOI] [PubMed] [Google Scholar]
- Hawker G. A., Mian S., Kendzerska T., French M. (2011). Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Research (Hoboken), 63 (Suppl. 11), S240–S252. 10.1002/acr.20543. [DOI] [PubMed] [Google Scholar]
- Katz J., Melzack R. (2011). The McGill Pain Questionnaire: Development, psychometric properties, and usefulness of the long-form, short-form and short-form-2 (3rd ed.). New York: Guilford Press. [Google Scholar]
- Kendall S., Holm N. R., Hojsted J., Frich L., Rotboll Nielsen P., Jensen N. H., Sjogren P. (2013). Chronic pain and the development of a symptom checklist: a pilot study. Acta Anaesthesiologica Scandinavica, 57(7), 920–928. 10.1111/aas.12137. [DOI] [PubMed] [Google Scholar]
- Kovacic K., Williams S., Li B. U., Chelimsky G., Miranda A. (2013). High prevalence of nausea in children with pain-associated functional gastrointestinal disorders: Are Rome criteria applicable? Journal of Pediatric Gastroenterology and Nutrition, 57(3), 311–315. 10.1097/MPG.0b013e3182964203. [DOI] [PubMed] [Google Scholar]
- Malaty H. M., Abudayyeh S., O'Malley K. J., Wilsey M. J., Fraley K., Gilger M. A., Rabeneck L. (2005). Development of a multidimensional measure for recurrent abdominal pain in children: population-based studies in three settings. Pediatrics, 115(2), e210–215. 10.1542/peds.2004-1412. [DOI] [PubMed] [Google Scholar]
- Mohammad S., Di Lorenzo C., Youssef N. N., Miranda A., Nurko S., Hyman P., Saps M. (2013). Assessment of abdominal pain through global outcomes and recent FDA recommendations in children: Are we ready for change? Journal of Pediatric Gastroenterology and Nutrition, 58, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnikes H. (2011). Quality of life in patients with irritable bowel syndrome. Journal of Clinical Gastroenterology, 45(Suppl.), S98–S101. 10.1097/MCG.0b013e31821fbf44. [DOI] [PubMed] [Google Scholar]
- Morren M., van Dulmen S., Ouwerkerk J., Bensing J. (2009). Compliance with momentary pain measurement using electronic diaries: A systematic review. European Journal of Pain, 13(4), 354–365. 10.1016/j.ejpain.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Ramaswami U., Stull D. E., Parini R., Pintos-Morell G., Whybra C., Kalkum G., Wiklund I. (2012). Measuring patient experiences in Fabry disease: Validation of the Fabry-specific Pediatric Health and Pain Questionnaire (FPHPQ). Health Quality Life Outcomes, 10, 116. 10.1186/1477-7525-10-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentz A. M., Kahrilas P., Stanghellini V., Tack J., Talley N. J., de la Loge C., Revicki D. A. (2004). Development and psychometric evaluation of the patient assessment of upper gastrointestinal symptom severity index (PAGI-SYM) in patients with upper gastrointestinal disorders. Quality of Life Research, 13(10), 1737–1749. [DOI] [PubMed] [Google Scholar]
- Sandler R. S., Everhart J. E., Donowitz M., Adams E., Cronin K., Goodman C., Rubin R. (2002). The burden of selected digestive diseases in the United States. Gastroenterology, 122(5), 1500–1511. [DOI] [PubMed] [Google Scholar]
- Stinson J. N. (2009). Improving the assessment of pediatric chronic pain: Harnessing the potential of electronic diaries. Pain Research & Management, 14(1), 59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talley N. J., Boyce P. M., Owen B. K., Newman P., Paterson K. J. (1995). Initial validation of a bowel symptom questionnaire and measurement of chronic gastrointestinal symptoms in Australians. Australia and New Zealand Journal of Medicine, 25(4), 302–308. [DOI] [PubMed] [Google Scholar]
- U.S. Public Health Service Act. (2010). Washington, DC: Government Printing Office. [Google Scholar]
- Wiklund I. K., Fullerton S., Hawkey C. J., Jones R. H., Longstreth G. F., Mayer E. A., Naesdal J. (2003). An irritable bowel syndrome-specific symptom questionnaire: Development and validation. Scandinavian Journal of Gastroenterology, 38(9), 947–954. [DOI] [PubMed] [Google Scholar]
- Yacob D., Di Lorenzo C., Bridge J. A., Rosenstein P. F., Onorato M., Bravender T., Campo J. V. (2013). Prevalence of pain-predominant functional gastrointestinal disorders and somatic symptoms in patients with anxiety or depressive disorders. Journal of Pediatrics, 163(3), 767–770. 10.1016/j.jpeds.2013.02.033. [DOI] [PubMed] [Google Scholar]