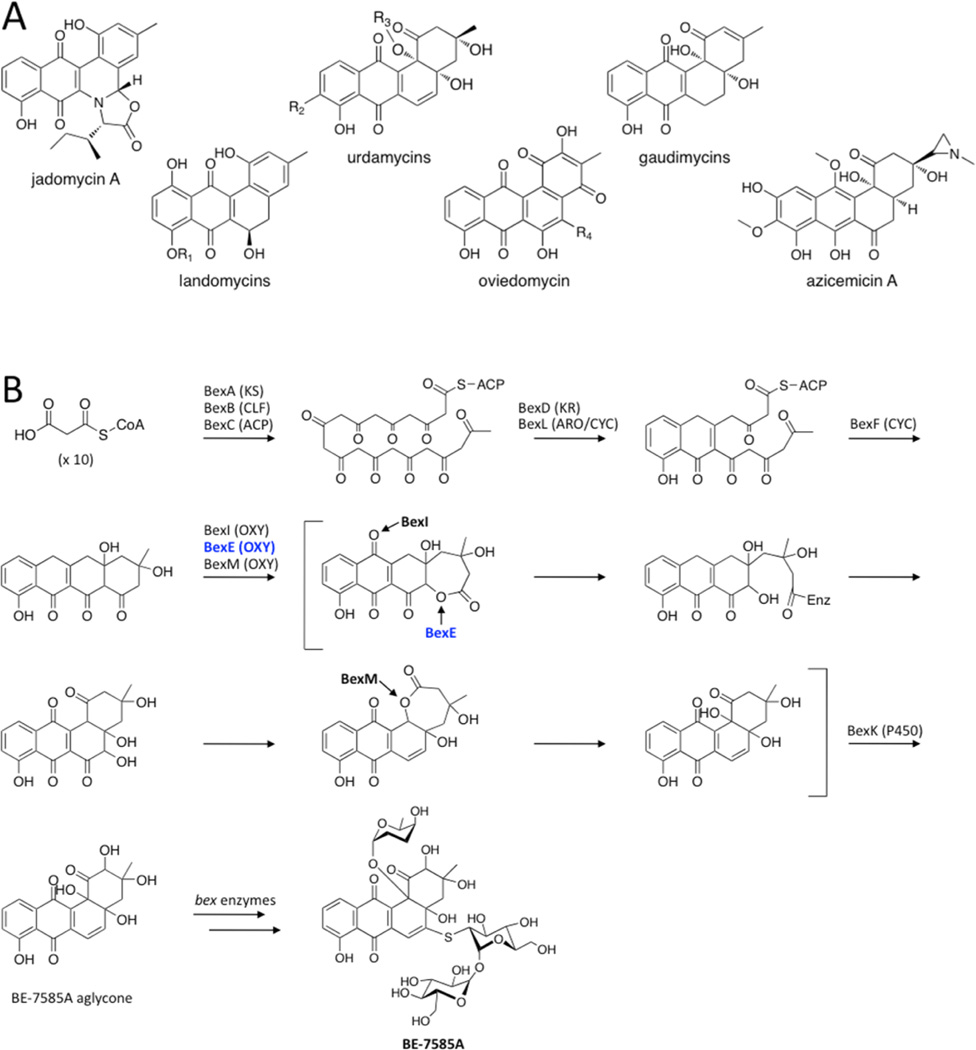
Figure 1.
(A) A representative set of angucycline type II polyketides. (B) The previously proposed biosynthesis of BE-7585A based on13C labeling studies, with an emphasis on the oxidation reactions.13 The minimal PKS produces a 20-carbon poly-β-ketone that is reduced at the C9 position and cyclized. BexF is proposed to catalyze fourth ring cyclization to produce the linear tetracyclic intermediate that is the substrate for a series of putative oxidation steps. In this work, we propose that a different linear tetracyclic intermediate, 12-deoxy-aklaviketonic acid, is the true BexE substrate. BexE, BexI, and BexM are proposed to form the BE-7585A aglycone core, which is further modified by hydroxylation and glycosylation to yield the final product.