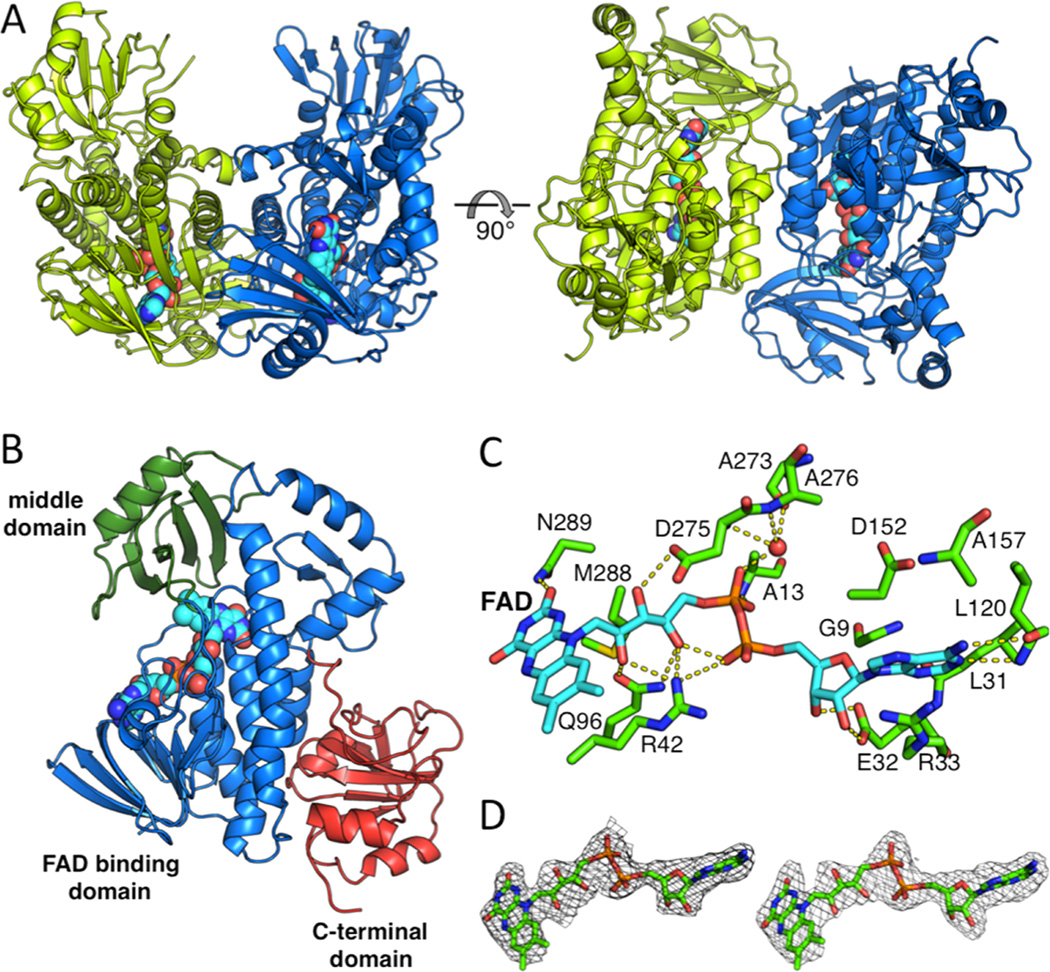
Figure 2.
Overall structure of the BexE dimer and domain organization of the BexE monomer. (A) The BexE dimer (monomer A in green and monomer B in blue). (B) The BexE monomer is composed of three domains: the FAD binding domain (residues 1–169 and 259–372, colored in blue), the middle domain (residues 170–258, colored in green), and the C-terminal domain (373–487, colored in red). (C) A molecular view of the BexE FAD binding site. (D) SA-Fo-Fc omit map contoured at 1.5 σ displaying clear density for FAD in monomer A (left) and monomer B (right).