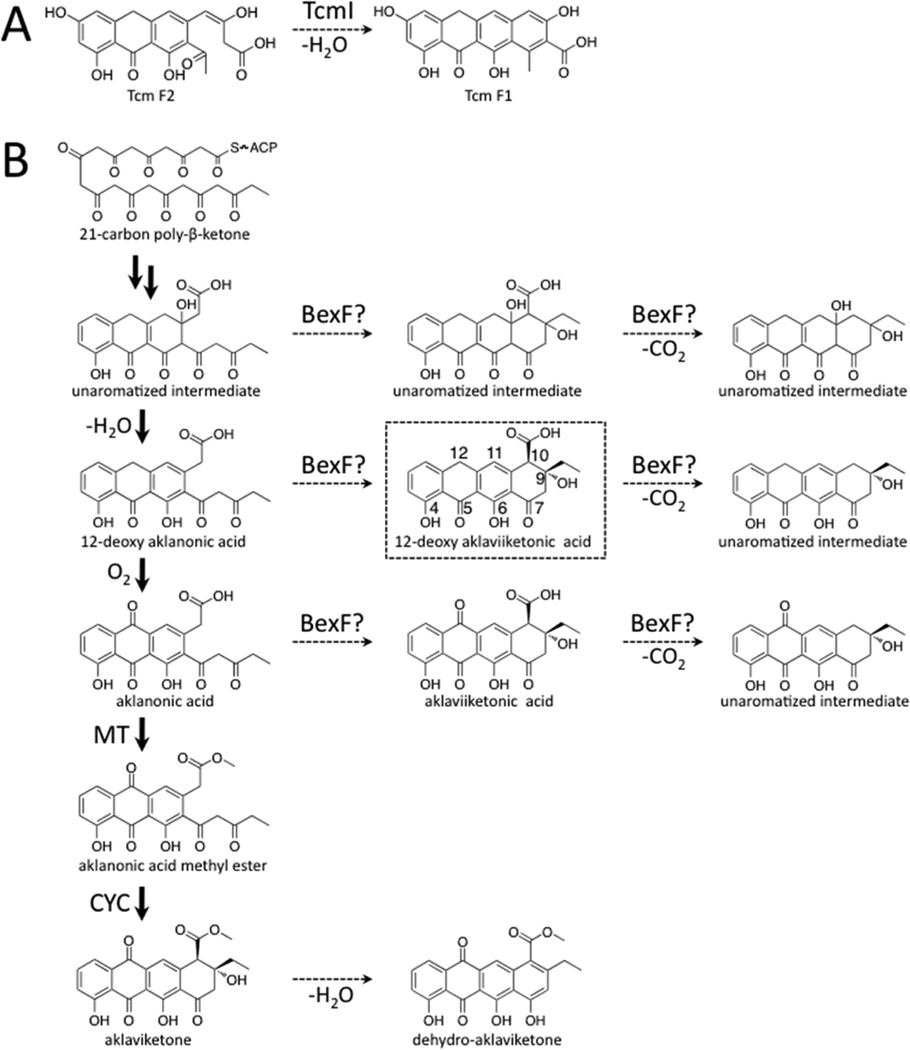
Figure 4.
Reaction catalyzed by TcmI and type II polyketide biosynthesis of aklaviketone in Streptomyces galilaeus (ATCC 31615). (A) TcmI is a fourth ring cyclase from the tetracenomycin biosynthesis pathway that catalyzes closure of the fourth ring of Tcm F2 to form Tcm F1. (B) Aklaviketone biosynthesis in Streptomyces galilaeus (ATCC 31615) follows a linear pathway starting from a 21-carbon intermediate (shown in bold arrows). Many putative intermediates occur transiently during aklaviketone biosynthesis, and we hypothesize that BexF may cyclize an intermediate to form the BexE substrate. Based on molecular docking studies, we propose that BexF generates 12-deoxy-aklaviketonic acid, which is then used by BexE as a substrate.