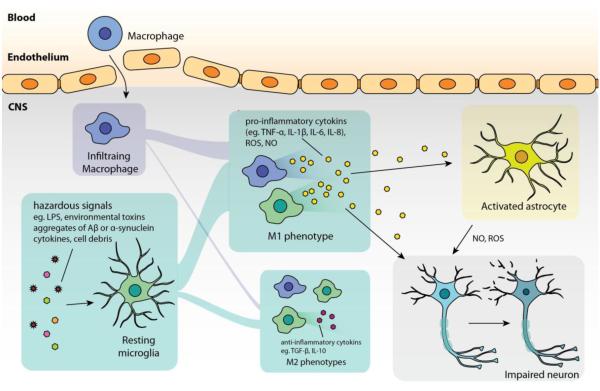
Figure 1. Common features in neuroinflammation.
In general, microglial cells are initially activated by hazardous signals, such as damage-associated molecular pattern molecules and cell debris from damaged cells in acute inflammation, aggregates of Aβ in Alzheimer’s disease, α-synuclein aggregates from abnormal dopaminergic neurons in Parkinson’s disease, SOD1 aggregates from diseased neuron in amyotrophic lateral sclerosis. In such pathologies, microglia (together with infiltrating macrophages) tend to polarize to M1 phenotype that amplifies neuroinflammation by producing pro-inflammatory cytokines (TNF-α, IL-1β, IL-6) as well as ROS and NO which lead to neuronal injury. Some pro-inflammatory cytokines (such as TNF-α, IL-1β) can also activate astrocytes to induce over-production of ROS and NO that exacerbate the injury. The impaired neurons are then susceptible to undergo apoptosis or necrosis, which, in turn, release molecules that further activate microglia.