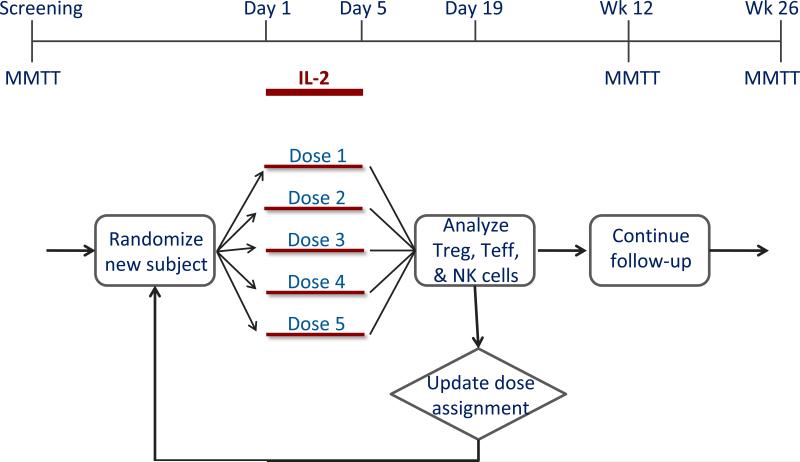
Fig. 2.
Proposed adaptive trial design for dose optimization of IL-2 in new-onset T1D. The adaptive trial is designed to determine an optimal dose using the continual reassessment method. Subject assignments to one of five IL-2 dose groups is based on ongoing flow cytometric analyses that quantify the relative expansion or activation of Treg, Teff, and NK cells. This design leads to significant efficiencies in terms of total recruitment required to determine an optimal dose. Reprinted with permission from Ehlers & Nepom [10].