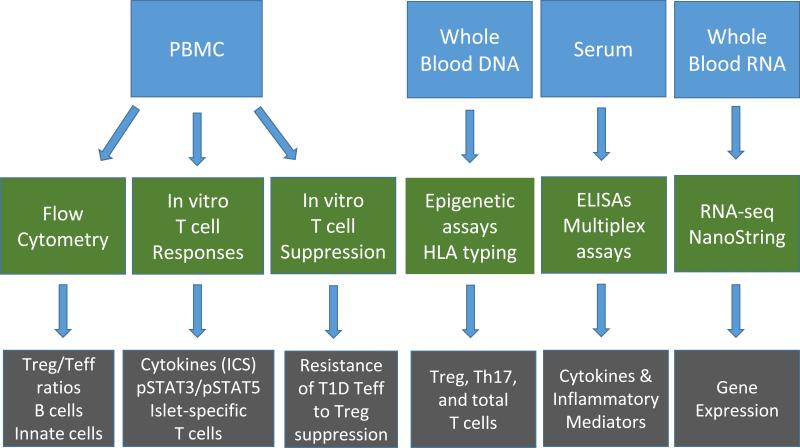
Fig. 3.
Standardized sample collection and mechanistic assay scheme to identify Immunologic surrogates of efficacy in T1D trials. Trials in new-onset T1D conducted by the Immune Tolerance Network (ITN) employ standardized sample collections (blue boxes: PBMCs, whole blood DNA, serum, and whole blood RNA), which are then analyzed by standardized assay procedures (green boxes: flow cytometry, in vitro T cell assays, epigenetic assays and genotyping, ELISAs and multiplex assays, and RNA-seq or NanoString analyses). Some of the expected outcomes of these assays are shown in the grey boxes.