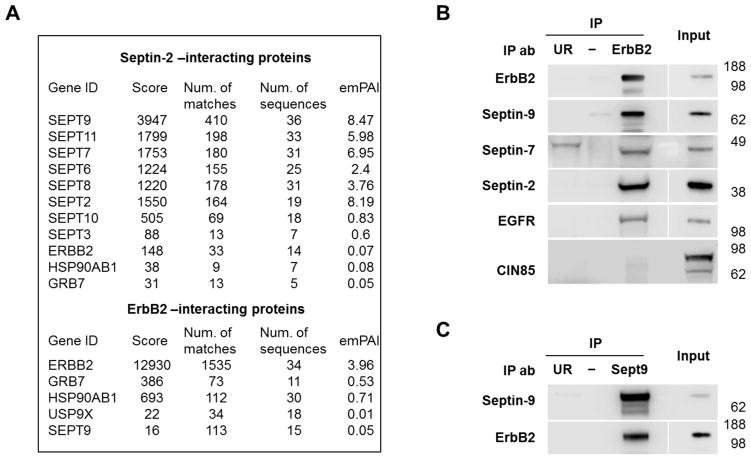
Figure 2. ErbB2 interacts with septins in HGE-20 gastric cancer cells.
Proteins immunoprecipitated using antibodies against septin-2 or ErbB2 from HGE-20 cell lysates were analyzed by mass spectrometry. ErbB2, known ErbB2-associated proteins, were detected in septin-2 immunoprecipitate and septin-9 was detected in ErbB2 immunoprecipitate (A). Interaction of ErbB2 with septins and a known dimerization partner, EGFR, was confirmed by western blot, following immunoprecipitation of ErbB2 in HGE-20 cell lysate. Ubiquitin ligase c-cbl binding protein, CIN85, a protein that mediates the interaction between septin-9 and EGFR, was not seen in ErbB2 IP (B). Interaction between septin-9 and ErbB2 was again confirmed by western blot following IP of HGE-20 cell lysates with septin-9 antibody (C). emPAI-exponentially modified protein abundance index, UR-unrelated antibody negative control (anti-human influenza hemagglutinin for ErbB2 IP and anti-green fluorescent protein for septin-9 IP).