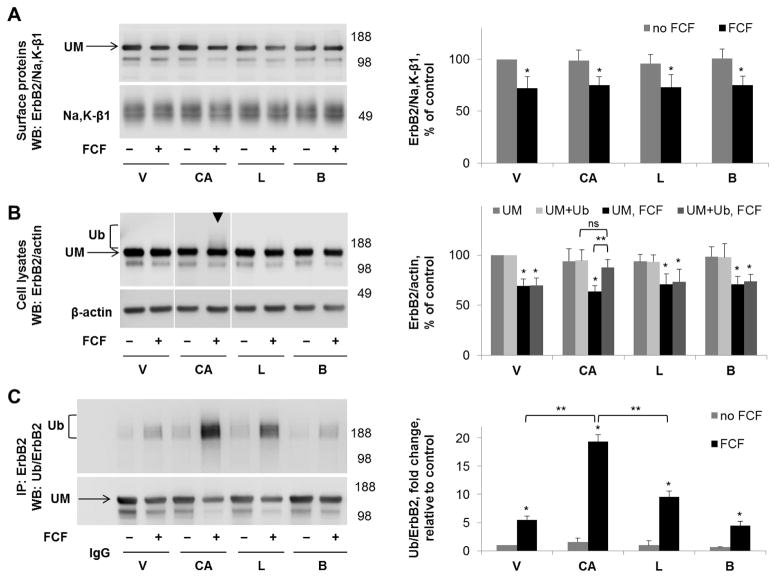
Figure 8. Cathepsin B inhibitor increases the amount of FCF-induced ubiquitylated forms of ErbB2 in cell lysates but not in the plasma membrane fraction.
HGE-20 cells were incubated with the indicated inhibitors for 6 hours, followed by basolateral biotinylation of surface proteins, streptavidin extraction, and western blot using anti-ErbB2 antibodies. Na+,K+-ATPase β1 subunit was used as a loading control. ErbB2 protein levels in the membrane fraction were not protected by any of the indicated inhibitors in the presence of FCF (A). In total cell lysates, ubiquitylated ErbB2, seen as an increased density running above the main ErbB2 band (arrowhead), was increased in the presence of cathepsin B inhibitor CA074-me (B). ErbB2 was immunoprecipitated, followed by western blot using anti-ubiquitin, then anti-ErbB2 antibodies, confirming a significant increase in ubiquitylated forms of ErbB2 in the presence of CA074-me and also showing a moderate increase in the presence of lactacystin (C). Quantification for each blot is shown to the right. Error bars, s.d., n=4 independent experiments, statistics done by Student’s t-test, ns-not significant, * - significant difference from the no-FCF control, P<0.05, ** - significant difference between indicated conditions, p<0.05, CA-CA074-me, L-lactacystin, B-bafilomycin, V-vehicle, Ub-ubiquitylated form of ErbB2, UM-unmodified form of ErbB2.