Abstract
Acute intermittent porphyria (AIP) is an inherited deficiency in the haem biosynthesis pathway. AIP is rare, affecting around 1 in 75 000 people. Acute attacks are characterised by abdominal pain associated with autonomic, neurological and psychiatric symptoms. AIP is rarely associated with posterior reversible encephalopathy syndrome (PRES). PRES is a clinicoradiological condition caused by the failure of the posterior circulation to autoregulate, resulting in cerebral oedema, headaches, nausea and seizures. This association is important because drugs used in the management of seizures may worsen an attack of AIP. This article describes a case of AIP and PRES in a young woman.
Background
Acute intermittent porphyria (AIP) is rare and the diagnosis is often delayed. Complications of AIP are diverse and include depression, neuropathic pain and seizures. In this case, AIP was diagnosed late in the patient's admission.
Awareness of the commonest presenting symptoms in AIP may increase vigilance in making the diagnosis. A case report of 108 participants from the USA identified abdominal pain as the most common presenting symptom. Individuals were most often female and between the ages of 20 and 40 years. Medication and surgery were the most common triggers.1
The association of abdominal pain and seizures in a young woman following surgery should trigger suspicion of AIP. AIP is rarely associated with posterior reversible encephalopathy syndrome (PRES).
Case presentation
A 26-year-old woman presented to the accident and emergency department, reporting of severe abdominal pain. Two weeks previously, she had undergone a gastric balloon insertion. Otherwise fit and well, she took no regular medications and had no medical history of note. She reported of diffuse, poorly localised abdominal pain, which she described as severe and unrelenting. On examination, she was in some distress; she was afebrile but tachycardic at 110 bpm. Her pulse was rapid but regular. Her observations were otherwise unremarkable. She had a soft, slightly distended abdomen with no localised guarding and no tenderness. Examination of the cardiovascular, respiratory and nervous system detected no abnormalities. She was reviewed by the surgical team and listed for emergency removal of her gastric balloon, and treated symptomatically with intravenous fluids and proton-pump inhibitors. Venous blood tests were performed and showed: Na+ 138 K+3.3 chloride 101 mmol/L, urea 5.3 mmol/L, creatinine 67 mmol/L, C reactive protein 1.5, magnesium 0.64 mmol/L, amylase 70, white cell count (WCC) 7.2, platelets 168×109/L. Following an uneventful procedure, the patient continued to suffer from abdominal pain. A CT of the abdomen was performed, which was unremarkable. The pain settled overnight and she was due to be discharged. However, while waiting for discharge, she suffered two tonic–clonic seizures in rapid succession and was started on a phenytoin infusion. A post seizure arterial blood gas showed: pH7.12, pCO2 5.7, pO2 55, base excess ± 2 mmol/L-12.4, K+3.3 and lactate of 10. The patient was reviewed by the medical registrar, who noted a new onset horizontal nystagmus. Neurological examination was otherwise normal. She continued to have a sinus tachycardia. She was treated with thiamine to cover a possible Wernicke's syndrome and with acyclovir and cefotaxime for encephalopathy. A CT of the head was performed, which demonstrated multiple hypoattenuation lesions in both cerebral hemispheres. A lumbar puncture revealed a white cell count of 2 mmol/L, red cell count 18 mmol/L, glucose 3.9 mmol/L. Cerebrospinal fluid cultures grew no organisms. In light of the CT changes, a brain MRI was performed. This demonstrated multifocal, non-enhancing areas of signal abnormality in both cerebral hemispheres (figure 1). These findings were non-specific, but suggestive of PRES. A cause for PRES was then sought: samples were sent for Nicotinic acetylcholine receptor antibodies, 5-hydroxyindoleacetic acid and metanephrines and a porphyria screen. Twenty-four hours later, the porphyria screen revealed raised urine porphyrins and urinary porphobilinogen (PBG). A diagnosis of AIP was made. The patient was started on a high carbohydrate diet and made a good recovery.
Figure 1.
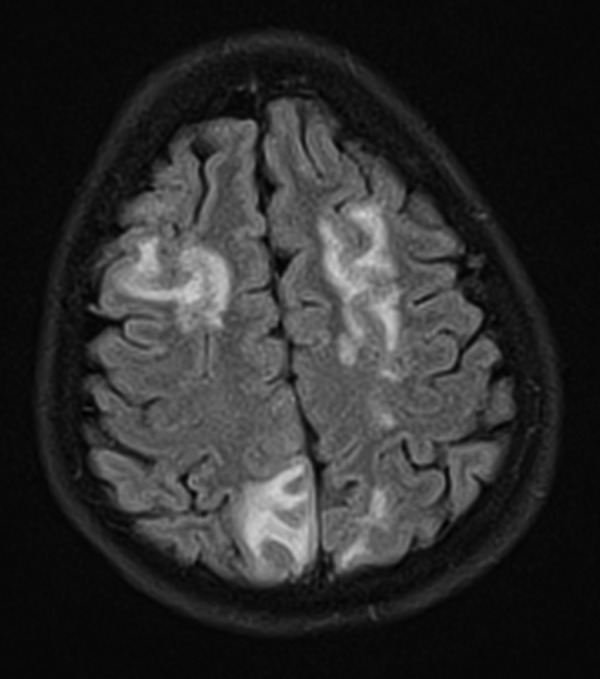
Diffuse T2-signal abnormalities produced by vasogenic oedema.
Differential diagnosis
The differential diagnosis of PRES includes:
Eclampsia
Severe hypertension
Systemic lupus erythematosus
Immunosuppressant therapy
Outcome and follow-up
After the diagnosis of AIP was made, all anticonvulsants were stopped. The patient was started on an infusion of intravenous dextrose and her symptoms rapidly settled. She was discharged after 3 days without symptoms and reviewed in the outpatient clinic. At review she remained asymptomatic.
Discussion
Abdominal pain has a wide range of possible causes; the physician must be aware of those rare but important causes of abdominal pain that may be missed.
AIP is one of a group of seven inherited disorders of haem synthesis. Symptoms include severe abdominal pain, constipation, autonomic nervous system disturbance and electrolyte disturbances. Attacks of porphyria can be precipitated by medication, menstruation and illness. It is caused by a deficiency of PBG deaminase, which leads to a build-up of PBG in the cytoplasm. Diagnosis is made by 24-hour urine collection, which will reveal markedly raised levels of PBG and urinary porphyrins during an acute attack.
Treatment of porphyria consists of a high carbohydrate diet supplemented with the use of intravenous glucose and haem-like substance infusions during acute attacks. These haem-like substances suppress 5-aminolevulinic acid synthase and the accumulation of haem precursors.
Management of seizures can actually worsen the condition. Many commonly used antiepileptics such as phenytoin and barbiturates will worsen symptoms. Levetiracetam is the preferred choice should seizure medication be required. Sufferers are advised to avoid drugs that precipitate attacks. In the long term, porphyria may lead to persistent neuromuscular weakness, high blood pressure and liver cirrhosis.
PRES has been reported in only a handful of patients with AIP.2–5 In this report, we have presented a new case of AIP with demonstrable PRES seen on MRI. PRES was first described in 1996, by Hinchey et al, as a clinical and radiological entity characterised by reversible headache, seizures and visual loss accompanying oedema seen especially in the occipital and parietal lobes.6 Treatment of PRES depends on the underlying cause.7
PRES is often associated with hypertension causing a vasogenic oedema. However, our patient remained normotensive throughout. She did, however, display autonomic features: she was tachycardic throughout her admission. This is noteworthy because the mechanism of PRES in AIP is unclear and has been suggested to be due to hypertension owing to autonomic dysfunction. An alternative suggestion is that depletion of nitric oxide synthase leads to vasoconstriction and oedema. Nitric oxide synthase is a haemoprotein and levels are reduced in AIP.
It is possible that this patient's acute attack was a result of weight loss following gastric band implantation or a result of the stress caused by surgery.
Porphyria is an important differential diagnosis in the patient with unexplained abdominal pain. Cortical manifestations of AIP remain rare, but this case report adds to a handful of cases worldwide associating AIP with PRES. This diagnosis requires vigilance as treatment of seizures with antiepileptics may risk making the patient worse by precipitating the underlying disease.
Learning points.
Abdominal pain with neurological symptoms should prompt consideration of porphyria.
Rarely, porphyria is associated with posterior reversible encephalopathy syndrome.
Recognition of this association is important to prevent precipitating further attacks of porphyria by use of inappropriate drugs.
Footnotes
Contributors: AD began this case report, MJG helped with the literature review and drafting of the final manuscript. Both authors approved the final manuscript.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Bonkovsky HL, Maddukuri VC, Yazici C et al. Acute porphyrias in the USA: features of 108 subjects from porphyria consortium. Am J Med 2014;127: 1233–41. 10.1016/j.amjmed.2014.06.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Celik M, Forta H, Dalkiliç T et al. MRI reveals reversible lesions resembling posterior reversible encephalopathy in porphyria. Neuroradiology 2002;44:839–41. 10.1007/s00234-002-0823-x [DOI] [PubMed] [Google Scholar]
- 3.King P, Bragdon A. MRI reveals multiple reversible cerebral lesions in an attack of acute intermittent porphyria. Neurology 1991;41:1300–2. [DOI] [PubMed] [Google Scholar]
- 4.Garg RK. Acute intermittent porphyria: a cause of posterior leukoencephalopathy syndrome. J Assoc Physicians India 2000;48:658. [PubMed] [Google Scholar]
- 5.Shen FC, Hsieh CH, Huang CR et al. Acute intermittent porphyria presenting as acute pancreatitis and posterior reversible encephalopathy syndrome. Acta Neurol Taiwan 2008;17:177–83. [PubMed] [Google Scholar]
- 6.Pedraza R, Marik P, Varon P. Posterior reversible encephalopathy syndrome: a review. Crit Care Shock 2009;12:135–43. [Google Scholar]
- 7.Hinchey J, Chaves C, Appignani B et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med 1996;334:494–500. 10.1056/NEJM199602223340803 [DOI] [PubMed] [Google Scholar]


