Figure 1.
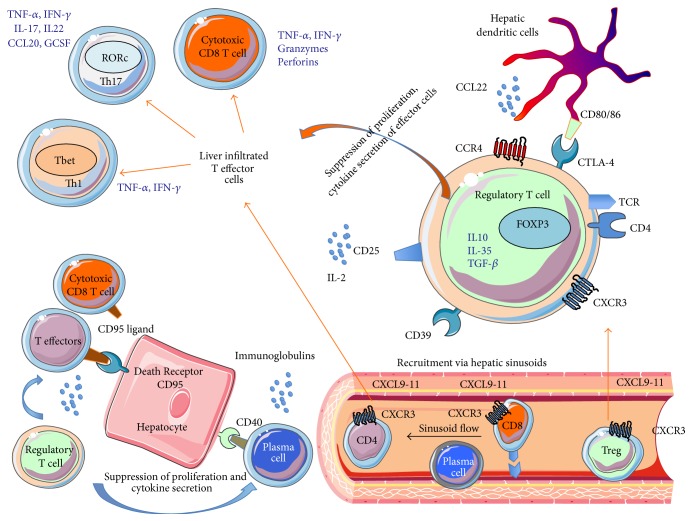
Pathogenesis of autoimmune hepatitis. Both effector T cells (CD4, CD8) and regulatory T cells (Treg) are recruited to inflamed autoimmune hepatitis liver via hepatic sinusoids. T effector cells lead to apoptosis of hepatocytes via CD95 ligand (dead ligand) expressed on them, which binds to CD95 on the hepatocytes. This killing action of T effector cells is regulated by regulatory T cells, which suppress proliferation and cytokine secretion of effector T cells. Plasma cells are also involved in immune-pathogenesis and they secrete immunoglobulin. Liver infiltrated T effector cells consist of Th17, Th1, and cytotoxic T cells. Th1 cells express T bet transcription factor; Th17 cells express transcription factor RORc. Cytotoxic T cells secrete IFN, TNF, granzymes, and perforins. Regulatory T cells (Treg = CD4CD25highCD127low) express liver tissue homing chemokine receptor CXCR3, which binds to its ligands CXCL9-11 expressed on inflamed hepatic sinusoid, hepatocytes, and bile ducts. Treg also expresses its functional markers CTLA4 (interacting with CD80/CD86 on dendritic cells). Dendritic cells secrete chemokine CCL22, which attracting chemokine receptor CCR4 expressing regulatory T cells. CD39 on the Treg can generate immunosuppressive adenosis from ATP in the hepatic microenvironment. IL-2, which acts on its receptor CD25, is crucial for intrahepatic Treg survival and function. TCR: T cell receptor.