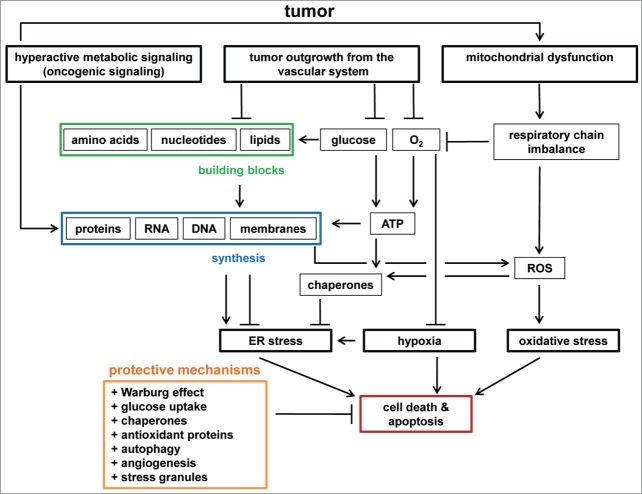
Figure 2.
Stresses in tumors. Hyperactive metabolic signaling (e.g., induced by oncogenes) can result in increased synthesis of proteins, RNA, DNA, and membranes. Lipid synthesis is required for ER homeostasis, whereas hyperactive protein synthesis can induce ER stress. Tumors eventually outgrow the vascular system, leading to a shortage in glucose, oxygen, and building blocks (amino acids, nucleotides, lipids). Glucose is required for ATP synthesis and is a carbon source for building block synthesis. Lack of ATP and building blocks inhibits lipid biosynthesis and chaperone activity. Therefore, ATP depletion enhances ER stress. Oxygen is required for ATP synthesis, and oxygen depletion results in hypoxia. ROS induce oxidative stress and originate from dysfunctions in mitochondria, for example triggered by oncogenic signaling and mtDNA damage, respiratory chain imbalances, and lipid and protein biosynthesis. ER stress, hypoxia, and oxidative stress induce stress responses to restore cellular homeostasis, and eventually trigger apoptosis. Cancer cells have protective mechanisms to prevent the induction of apoptosis by chronic stresses. Examples of such mechanisms are metabolic transformation (the Warburg effect), glucose uptake, chaperone and antioxidant protein synthesis, autophagy, angiogenesis, and stress granule formation.